168521
Luperox® DI, tert-Butyl peroxide
98%
Synonym(s):
tert-Butyl peroxide, Di-tert-butyl peroxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
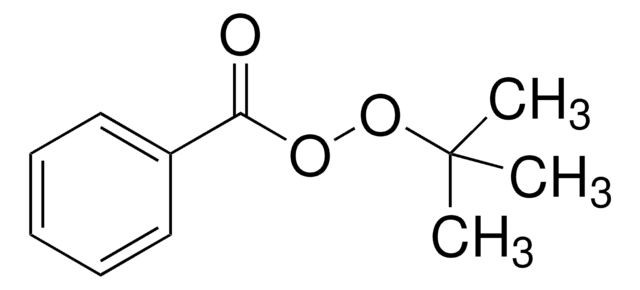
Linear Formula:
(CH3)3COOC(CH3)3
CAS Number:
Molecular Weight:
146.23
Beilstein:
1735581
EC Number:
MDL number:
UNSPSC Code:
12352120
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
40 mmHg ( 20 °C)
Quality Level
Assay
98%
form
liquid
reaction suitability
reagent type: oxidant
refractive index
n20/D 1.3891 (lit.)
bp
109-110 °C (lit.)
density
0.796 g/mL at 25 °C (lit.)
functional group
peroxide
storage temp.
2-8°C
SMILES string
CC(C)(C)OOC(C)(C)C
InChI
1S/C8H18O2/c1-7(2,3)9-10-8(4,5)6/h1-6H3
InChI key
LSXWFXONGKSEMY-UHFFFAOYSA-N
Application
Luperox®DI, tert-Butyl peroxide has been used as a radical initiator to induce free radical polymerization. It has also been used as a cetane enhancer in a study to determine the phase behavior of carboxylate-based extended surfactant reverse micellar microemulsions with ethanol and vegetable oil/diesel blends.
Legal Information
Product of Arkema Inc.
Luperox is a registered trademark of Arkema Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Flam. Liq. 2 - Muta. 2 - Org. Perox. E
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 1
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vegetable oil-based microemulsions using carboxylate-based extended surfactants and their potential as an alternative renewable biofuel.
Attaphong C
Fuel: The Science and Technology of Fuel and Energy, 94, 606-613 (2012)
Geun-Tae Yun et al.
Science advances, 4(8), eaat4978-eaat4978 (2018-08-29)
Both high static repellency and pressure resistance are critical to achieving a high-performance omniphobic surface. The cuticles of springtails have both of these features, which result from their hierarchical structure composed of primary doubly reentrant nanostructures on secondary microgrooves. Despite
A Mortensen et al.
FEBS letters, 426(3), 392-396 (1998-05-26)
Peroxyl radicals, as model for peroxyl radicals formed during autoxidation of lipids, have been generated in three solvent systems (cyclohexane, tetrahydrofuran and tert-butanol/water) by steady-state and laser flash photolysis, and their reaction with beta-carotene studied. Steady-state photolysis experiments showed that
Free radical polymerization of caffeine?containing methacrylate monomers.
Nelson AM
Journal of Polymer Science Part A: Polymer Chemistry, 53(24), 2829-2837 (2015)
Hironori Kitaguchi et al.
Journal of the American Chemical Society, 127(18), 6605-6609 (2005-05-05)
Well-resolved ESR spectra of free pentadienyl radicals have been observed under photoirradiation of di-tert-butylperoxide (Bu(t)OOBu(t)) and polyunsaturated fatty acids in the absence of O(2), allowing us to determine the hfc values. The hfc values of linoleyl radical indicate that the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service