16120
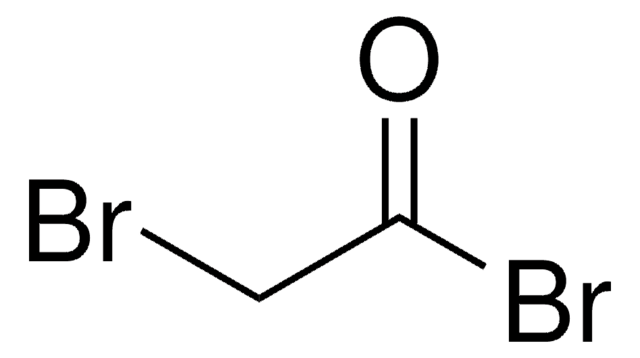
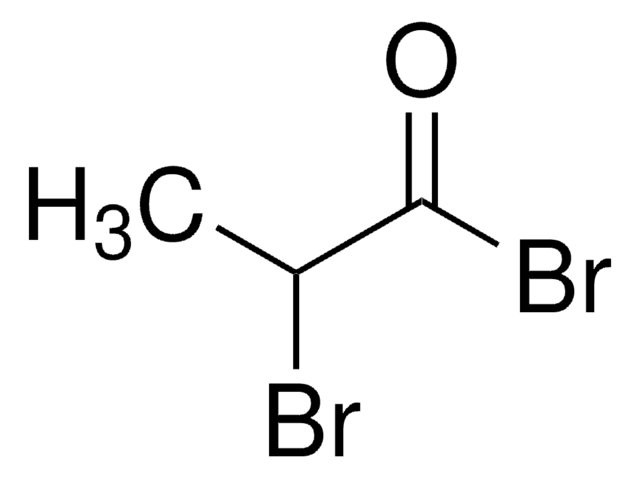
Bromoacetyl chloride
≥95% (GC)
Synonym(s):
2-Bromo-1-chloro-ethanal, 2-Bromoacetyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2COCl
CAS Number:
Molecular Weight:
157.39
Beilstein:
1209323
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95% (GC)
refractive index
n20/D 1.495
bp
127-128 °C (lit.)
density
1.89 g/mL at 20 °C
functional group
acyl chloride
bromo
storage temp.
2-8°C
SMILES string
ClC(=O)CBr
InChI
1S/C2H2BrClO/c3-1-2(4)5/h1H2
InChI key
SYZRZLUNWVNNNV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The competitive photodissociation of bromoacetyl chloride has been studied by ab initio methods.
Application
Bromoacetyl chloride was used in the synthesis of α,α-disubstituted thioisomünchnones. It was also used in the preparation of 1,3-dibromoacetone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cycloaddition chemistry of anhydro-4-hydroxy-1, 3-thiazolium hydroxides (thioisomunchnones) for the synthesis of heterocycles.
Padwa A, et al.
Synthesis, 1994(9), 993-1004 (1994)
Non-adiabatic effects in the photodissociation of bromoacetyl chloride.
Bacchus-Montabonel M-C, et al.
Chemical Physics Letters, 374(3), 307-313 (2003)
Xueqi Sun et al.
International journal of biological macromolecules, 152, 349-358 (2020-02-23)
In this study, a new class of chitosan derivatives possessing sulfonium salts was synthesized, and characterized by FT-IR, 1H NMR, 13C NMR, and elemental analyses. IR spectra, 1H NMR and 13C NMR of the structural units of these polymers validated
S S Husain et al.
The Biochemical journal, 108(5), 855-859 (1968-08-01)
Papain was irreversibly inhibited by 1,3-dibromoacetone, a reagent designed to react first with the active-site cysteine residue and subsequently with a second nucleophile. The molecular weight of the inhibited enzyme was indistinguishable from that of papain itself, and no evidence
Jonathan E Meegan et al.
Soft matter, 13(35), 5922-5932 (2017-08-05)
Four novel amino acid-functionalised triphenylenes have been prepared with glycine, l-alanine, l-phenylalanine and l-tryptophan ethyl ester side-chains. The glycine derivative is a good gelator of chloroform, the alanine derivative gels ethanol and toluene, and the phenylalanine derivative gels benzene and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service