145610
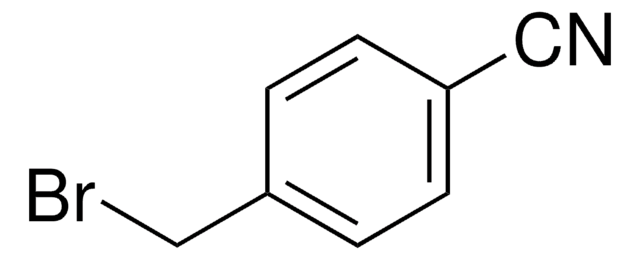
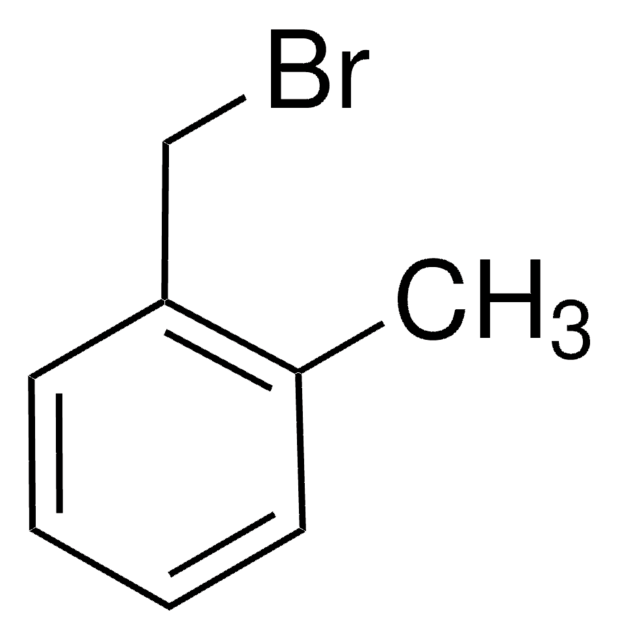
3-(Bromomethyl)benzonitrile
95%
Synonym(s):
α-Bromo-m-tolunitrile, 3-Cyanobenzyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2C6H4CN
CAS Number:
Molecular Weight:
196.04
Beilstein:
742356
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
93-96 °C
functional group
bromo
nitrile
SMILES string
BrCc1cccc(c1)C#N
InChI
1S/C8H6BrN/c9-5-7-2-1-3-8(4-7)6-10/h1-4H,5H2
InChI key
CVKOOKPNCVYHNY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-(Bromomethyl)benzonitrile undergoes Suzuki cross-coupling reaction with bis(pinacolato)diboron.
Application
3-(Bromomethyl)benzonitrile was used in the synthesis of 3-(bromomethyl)benzaldehyde.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lydia C Gilday et al.
Dalton transactions (Cambridge, England : 2003), 41(23), 7092-7097 (2012-05-09)
Triazole and triazolium groups have been integrated into a zinc(II) metalloporphyrin-based structural framework to produce two porphyrin-cages for anion sensing applications. UV/visible spectroscopic titration investigations reveal both host systems exhibit strong anion binding affinities, with the positively-charged triazolium-porphyrin cage capable
Synthesis of benzylic boronates via palladium-catalyzed cross-coupling reaction of bis (pinacolato) diboron with benzylic halides.
Giroux A.
Tetrahedron Letters, 44(2), 233-235 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service