133159
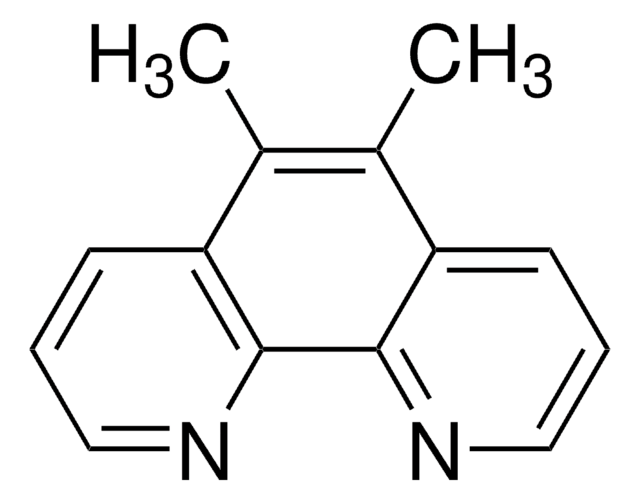
Bathophenanthroline
97%
Synonym(s):
4,7-Diphenyl-1,10-phenanthroline, BPhen
About This Item
Recommended Products
Assay
97%
form
solid
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
218-220 °C (lit.)
Orbital energy
HOMO 6.4 eV
LUMO 3 eV
OLED Device Performance
ITO/MoO3/NPD/Alq3/BPhen/LiF/Al
ITO/NPD/Alq3/BPhen/LiF/Al
ITO/PEDOT:PSS/NPD/Alq3/BPhen/LiF/Al
ITO/PEDOT:PSS/PVK:Ir(mppy)2 (6wt%)/BPhen/LiF:Al
ITO/TCTA/Ir(ppy)3/BPhen/LiF/Al
PEDOT:PSS/MoO3/NPD/Alq3/BPhen/LiF/Al
greener alternative category
SMILES string
c1ccc(cc1)-c2ccnc3c2ccc4c(ccnc34)-c5ccccc5
InChI
1S/C24H16N2/c1-3-7-17(8-4-1)19-13-15-25-23-21(19)11-12-22-20(14-16-26-24(22)23)18-9-5-2-6-10-18/h1-16H
InChI key
DHDHJYNTEFLIHY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis and luminescent properties of a ternary erbium (III) complex: A study on the luminescent properties of an erbium(III) complex with bathophenanthroline, which may have implications for materials science and sensor applications (Martín-Ramos et al., 2015).
- Aggregation induced emission enhancement from Bathophenanthroline microstructures: Investigation of bathophenanthroline microstructures that exhibit enhanced emission properties and potential use as sensors for detecting mercury ions in water (Mazumdar et al., 2014).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Since their discovery, organic light emitting devices (OLEDs) have evolved from a scientific curiosity into a technology with applications in flat panel displays and the potential to revolutionize the lighting market. During their relatively short history, the technology has rapidly advanced, and device efficiencies have increased more than 20-fold, approaching the theoretical limit for internal quantum efficiencies.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service