130176
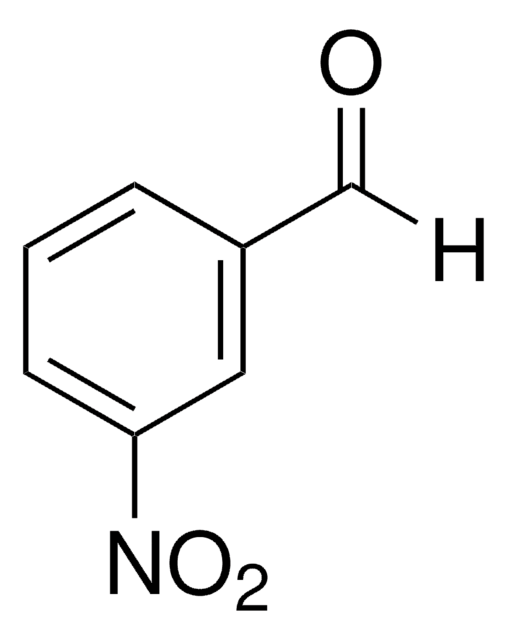
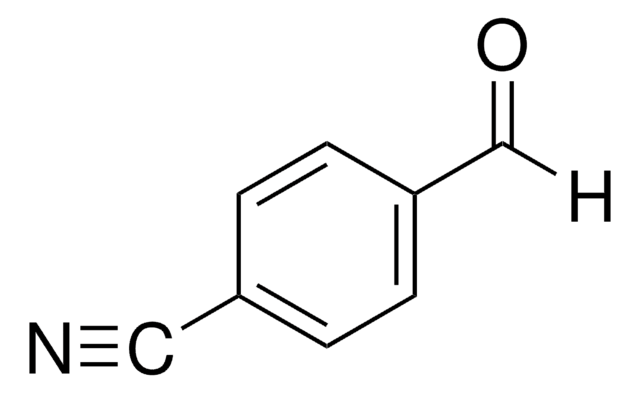
4-Nitrobenzaldehyde
98% (GC)
Synonym(s):
p-Nitrobenzaldehyde
About This Item
Recommended Products
Quality Level
Assay
98% (GC)
mp
103-106 °C (lit.)
functional group
aldehyde
nitro
SMILES string
[O-][N+](=O)c1ccc(C=O)cc1
InChI
1S/C7H5NO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5H
InChI key
BXRFQSNOROATLV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 130176-100G | 4061835281404 |
| 130176-25G | 4061835281411 |
| 130176-10G | 4061838727282 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service