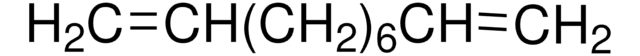All Photos(1)
About This Item
Linear Formula:
H2C=CHCH2CH(OH)CH=CH2
CAS Number:
Molecular Weight:
98.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.448 (lit.)
density
0.878 g/mL at 25 °C (lit.)
functional group
allyl
hydroxyl
SMILES string
OC(CC=C)C=C
InChI
1S/C6H10O/c1-3-5-6(7)4-2/h3-4,6-7H,1-2,5H2
InChI key
SZYLTIUVWARXOO-UHFFFAOYSA-N
General description
1,5-Hexadien-3-ol is the starting reagent for the enantioselective synthesis of (+)-rogioloxepane A. It undergoes Sharpless kinetic resolution and ring-closing metathesis to yield nine membered oxocene.
Application
1,5-Hexadien-3-ol was used in the synthesis of (+)-obtusenyne.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
84.2 °F - closed cup
Flash Point(C)
29 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Induction of rejection of successful allografts of rat islets by donor peritoneal exudate cells.
P E Lacy et al.
Transplantation, 28(5), 415-420 (1979-11-01)
Michael T Crimmins et al.
Journal of the American Chemical Society, 125(25), 7592-7595 (2003-06-19)
A total synthesis of the laurencia metabolite (+)-obtusenyne has been completed. The key steps include a Sharpless kinetic resolution and an asymmetric glycolate alkylation to establish the stereogenic centers adjacent to the ether linkage and a ring-closing metathesis reaction to
Yan-Lun Ju et al.
Plant physiology and biochemistry : PPB, 130, 501-510 (2018-08-11)
Grapes are one of the most important fruits because of their economic and nutritional benefits, and grapevines are widely cultivated in arid and semi-arid areas. Therefore, it is critical to study the mechanism by which grapevines respond to water stress.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service