116068
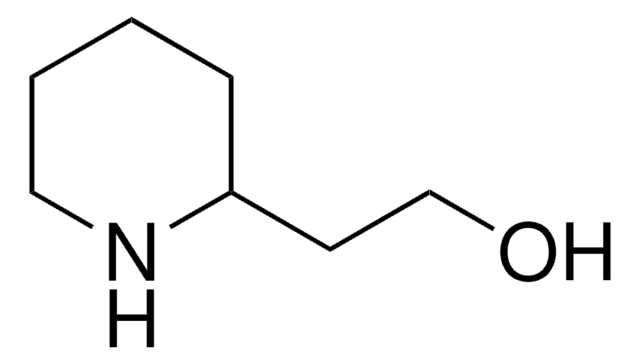
1-(2-Hydroxyethyl)piperidine
ReagentPlus®, 99%
Synonym(s):
1-Piperidineethanol, 2-Piperidinoethanol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H15NO
CAS Number:
Molecular Weight:
129.20
Beilstein:
103390
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
refractive index
n20/D 1.4804 (lit.)
bp
199-202 °C (lit.)
density
0.973 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OCCN1CCCCC1
InChI
1S/C7H15NO/c9-7-6-8-4-2-1-3-5-8/h9H,1-7H2
InChI key
KZTWONRVIPPDKH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-(2-Hydroxyethyl)piperidine (1-Piperidineethanol) has been used as the base quencher to determine the efficiency of the decomposition of photoacid generators.
Counter-ion used for investigations of skin permeation of flurbiprofen
Catalyst used for enantioselective synthesis
Amine quencher used during synthesis of photoacid generators for ArF lithography
Adsorbent for capture and release of pressurized carbon dioxide for greenhouse gas control
Catalyst used for enantioselective synthesis
Amine quencher used during synthesis of photoacid generators for ArF lithography
Adsorbent for capture and release of pressurized carbon dioxide for greenhouse gas control
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
François Lutz et al.
Journal of the American Chemical Society, 127(35), 12206-12207 (2005-09-01)
An asymmetric autocatalytic reaction has been catalyzed by a mixture of chiral and achiral beta-amino alcohols. The absolute configuration of the highly enantioenriched obtained product (>98% ee) was shown to depend not only on the absolute configuration of the chiral
A standard addition technique to quantify photoacid generation in chemically amplified photoresist.
Chemistry of Materials, 13(11), 4154-4162 (2001)
K. Tomizaki, et al.,
Chemistry Letters (Jpn), 37, 516-517 (2008)
T. Asakura, et al
J. Photopolym. Sci. Technol., 18, 407-414 (2005)
Hongxia Cui et al.
Journal of pharmaceutical sciences, 104(10), 3395-3403 (2015-09-10)
The study was carried out to investigate the mechanism of the ion-pair strategy in modulating zaltoprofen (ZAL) skin permeability. Seven organic amines were chosen as counter ions and the formation of ion pairs was confirmed by Fourier transform infrared and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service