115983
Trimethyl 1,3,5-benzenetricarboxylate
98%
Synonym(s):
Trimesic acid trimethyl ester, Trimethyl trimesate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
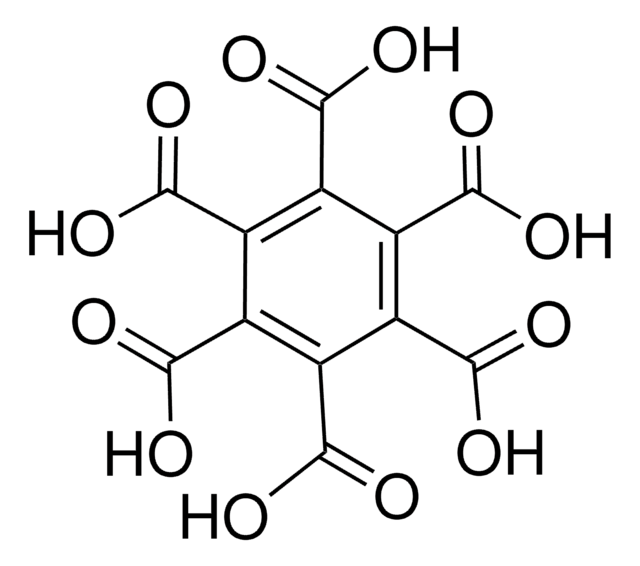
Linear Formula:
C6H3(CO2CH3)3
CAS Number:
Molecular Weight:
252.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
145-147 °C (lit.)
functional group
ester
SMILES string
COC(=O)c1cc(cc(c1)C(=O)OC)C(=O)OC
InChI
1S/C12H12O6/c1-16-10(13)7-4-8(11(14)17-2)6-9(5-7)12(15)18-3/h4-6H,1-3H3
InChI key
RGCHNYAILFZUPL-UHFFFAOYSA-N
Related Categories
Application
Trimethyl 1,3,5-benzenetricarboxylate has been used to synthesize yttrium trimesates with open frameworks.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bao-Xia Dong et al.
Dalton transactions (Cambridge, England : 2003), 39(24), 5683-5687 (2010-05-21)
Two novel yttrium trimesates with open frameworks have been synthesized by the self-assembly of trimethyl 1,3,5-benzenetricarboxylate and Y(3+) ion in mixed solvent of dimethylformamide (DMF)/water and diethylformamide (DEF)/water, where changes of solvents with different molecular sizes lead to the formation
Sheng-Mu You et al.
Nanomaterials (Basel, Switzerland), 10(9) (2020-09-02)
Photoelectrochemical (PEC) water splitting is a promising strategy to improve the efficiency of oxygen evolution reactions (OERs). However, the efficient adsorption of visible light as well as long-term stability of light-harvesting electrocatalysis is the crucial issue in PEC cells. Metal-organic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service