112968
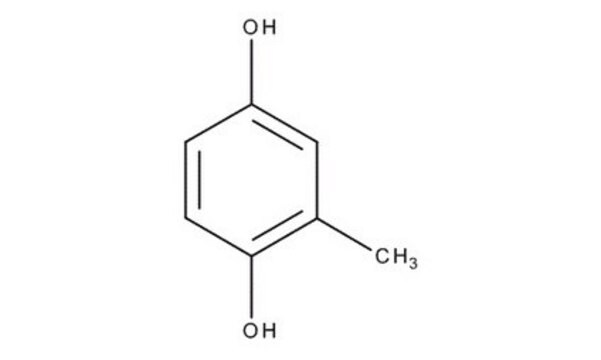
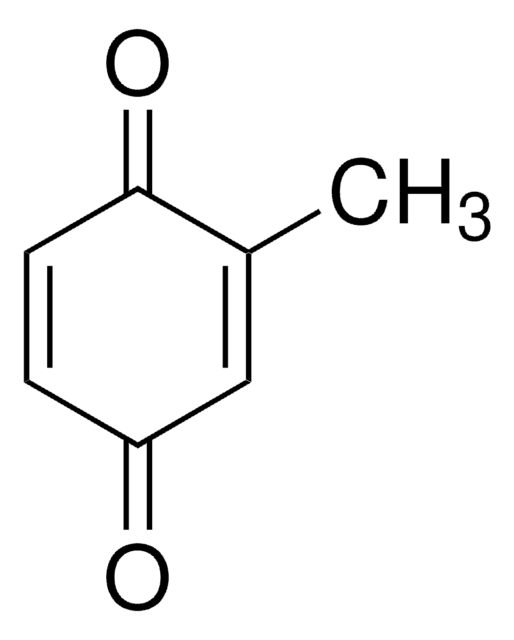
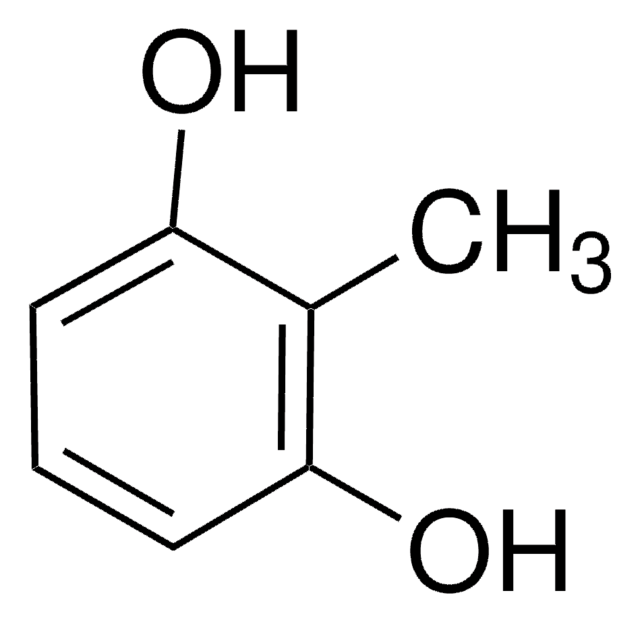
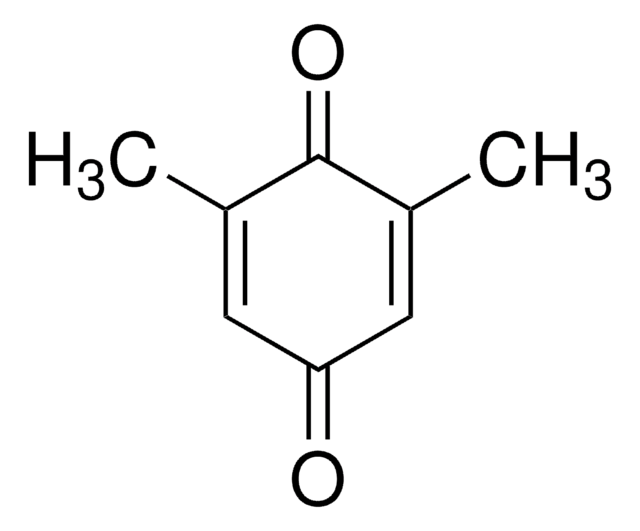
Methylhydroquinone
99%
Synonym(s):
Toluhydroquinone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3C6H3-1,4-(OH)2
CAS Number:
Molecular Weight:
124.14
Beilstein:
2041489
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
autoignition temp.
851 °F
mp
128-130 °C (lit.)
SMILES string
Cc1cc(O)ccc1O
InChI
1S/C7H8O2/c1-5-4-6(8)2-3-7(5)9/h2-4,8-9H,1H3
InChI key
CNHDIAIOKMXOLK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methylhydroquinone is produced by the oxidation of o-cresol by the mutants G103S, G103S/A107G, and G103S/A107T.
Application
Methylhydroquinone can be used as a reactant to prepare:
- A semiflexible thermotropic polyester via polycondensation reaction with 4,4′-sebacoyldioxydibenzoyl chloride.
- A sesquiterpene (±)-helibisabonol A.
- poly{hexakis[(methyl)(4-hydroxyphenoxy)]cyclotriphosphazene} by reacting with hexachlorocyclotriphosphazene.
- 6-Hydroxy-4,7-dimethyl-2H-1-benzopyran-2-one by treating with ethyl acetoacetate in the presence of H2SO4 as a catalyst.
Biochem/physiol Actions
Methylhydroquinone (Toluquinol) inhibits the growth of endothelial and tumor cells in culture in the micromolar range and is a promising drug candidate in the treatment of cancer and other angiogenesis-related pathologies.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1A - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
341.6 °F - closed cup
Flash Point(C)
172 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ying Tao et al.
Journal of bacteriology, 186(14), 4705-4713 (2004-07-03)
Wild-type toluene 4-monooxygenase (T4MO) of Pseudomonas mendocina KR1 oxidizes toluene to p-cresol (96%) and oxidizes benzene sequentially to phenol, to catechol, and to 1,2,3-trihydroxybenzene. In this study T4MO was found to oxidize o-cresol to 3-methylcatechol (91%) and methylhydroquinone (9%), to
Stefanie Töwe et al.
Molecular microbiology, 66(1), 40-54 (2007-08-30)
Catechol and 2-methylhydroquinone (2-MHQ) cause the induction of the thiol-specific stress response and four dioxygenases/glyoxalases in Bacillus subtilis. Using transcription factor arrays, the MarR-type regulator YkvE was identified as a repressor of the dioxygenase/glyoxalase-encoding mhqE gene. Transcriptional and proteome analyses
C Schewe et al.
Biomedica biochimica acta, 50(3), 299-305 (1991-01-01)
Various polymeric oxidation products of polyphenols strongly inhibited the purified lipoxygenase of rabbit reticulocytes, whereas the prostaglandin H synthase of sheep vesicular gland was only weakly inhibited. The oxidized polymeric preparations of caffeic acid, 2,5-dihydroxytoluene and 3,4-dihydroxytoluene were the most
Van Duy Nguyen et al.
Proteomics, 7(9), 1391-1408 (2007-04-05)
Bacillus subtilis is exposed to a variety of antimicrobial compounds in the soil. In this paper, we report on the response of B. subtilis to the fungal-related antimicrobials 6-brom-2-vinyl-chroman-4-on (chromanon) and 2-methylhydroquinone (2-MHQ) using proteome and transcriptome analyses. Chromanon, a
C Furihata et al.
Japanese journal of cancer research : Gann, 84(3), 223-229 (1993-03-01)
The possible tumor-promoting and tumor-initiating activities of p-methylcatechol and methylhydroquinone in the pyloric mucosa of male F344 rats were studied. Ornithine decarboxylase (ODC) and replicative DNA synthesis (RDS) were used as markers of tumor promotion and DNA single strand scission
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service