110213
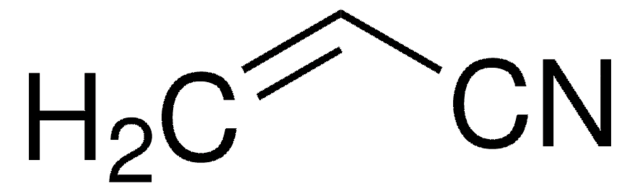
Acrylonitrile
≥99%, contains 35-45 ppm monomethyl ether hydroquinone as inhibitor
Synonym(s):
Vinyl cyanide
About This Item
Recommended Products
vapor density
1.83 (vs air)
Quality Level
vapor pressure
86 mmHg ( 20 °C)
Assay
≥99%
autoignition temp.
897 °F
contains
35-45 ppm monomethyl ether hydroquinone as inhibitor
expl. lim.
17 %
refractive index
n20/D 1.391 (lit.)
bp
77 °C (lit.)
mp
−83 °C (lit.)
SMILES string
C=CC#N
InChI
1S/C3H3N/c1-2-3-4/h2H,1H2
InChI key
NLHHRLWOUZZQLW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In the preparation of the 3D-printed polymer material, Acrylonitrile Butadiene Styrene (ABS) which is a commonly used engineering thermoplastic known for its high strength, durability, and heat resistance. It serves as a suitable substrate for a wide range of applications, including in the medical field, compatible manufacturing processes, injection molding, blow molding, and extrusion.
- In the copolymerization with lignosulfonate to develop a carbon fiber precursor. This copolymer can serve as a precursor material that undergoes further thermal treatment to produce carbon fibers.
- To synthesize acrylamide grafted acrylonitrile copolymer membranes, which serve as a support matrix for the immobilization of cellulase enzymes.
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Carc. 1B - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
23.0 °F - closed cup
Flash Point(C)
-5 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service