T9299
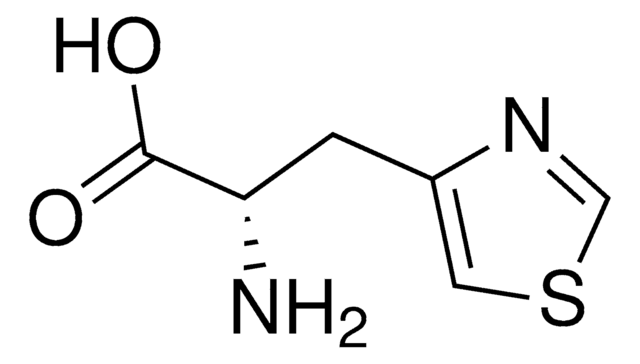
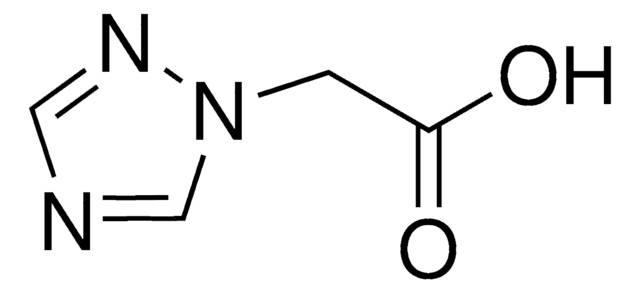
β-(1,2,4-Triazol-3-yl)-DL-alanine
≥98% (TLC)
Synonym(s):
(±)-2-Amino-3-(1,2,4-triazol-3-yl)propionic acid, DL-1,2,4-Triazole-3-alanine
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C5H8N4O2
CAS Number:
Molecular Weight:
156.14
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
β-(1,2,4-Triazol-3-yl)-DL-alanine,
Assay
≥98% (TLC)
Quality Level
form
powder
color
white to off-white
storage temp.
−20°C
SMILES string
NC(Cc1nc[nH]n1)C(O)=O
InChI
1S/C5H8N4O2/c6-3(5(10)11)1-4-7-2-8-9-4/h2-3H,1,6H2,(H,10,11)(H,7,8,9)
InChI key
CAPORZWUTKSILW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Linkage
Inhibitory histidine analog.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yutaka Ikeda et al.
Protein engineering, 16(9), 699-706 (2003-10-16)
Proteins containing unnatural amino acids have immense potential in biotechnology and medicine. We prepared several histidine analogues including a novel histidine analogue, beta-(1,2,3-triazol-4-yl)-DL-alanine. These histidine analogues were assayed for translational activity in histidine-auxotrophic Escherichia coli strain UTH780. We observed that
K A Sment et al.
Applied and environmental microbiology, 55(5), 1295-1297 (1989-05-01)
In contrast to wild-type cells, it was found that triazole-alanine-resistant mutants of Methanococcus voltae excreted histidine, proline, phenylalanine, and tyrosine in various combinations. These results suggest that a form of general amino acid biosynthetic control may operate in this methanogen.
S H Beiboer et al.
Protein engineering, 9(4), 345-352 (1996-04-01)
The effect of the substitution of the active site histidine 48 by the unnatural 1,2,4-triazole-3-alanine (TAA) amino acid analogue in porcine pancreas phospholipase A2 (PLA2) was studied. TAA was introduced biosynthetically using a his-auxotrophic Escherichia coli strain. To study solely
Younas Aouine et al.
Molecules (Basel, Switzerland), 16(4), 3380-3390 (2011-04-23)
A simple synthetic approach to racemic N-tert-butyloxycarbonyl-2-methyl-3-(1H-1,2,4-triazol-1-yl)alanine (5) in four steps and 68% overall yield starting from oxazoline derivative 1 is reported. This synthesis involves the alkylation of 1H-1,2,4-triazole with an O-tosyloxazoline derivative, followed by an oxazoline ring-opening reaction and
P Soumillion et al.
Protein engineering, 11(3), 213-217 (1998-06-05)
The only histidine residue in the H31N-H137N double mutant of phage lambda lysozyme (lambdaL), at position 48, was biosynthetically replaced by the analogue 1,2,4-triazole-3-alanine (Taz), the basicity of which is 3 pKa units lower. A histidine-auxotrophic strain was grown to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service