T3787
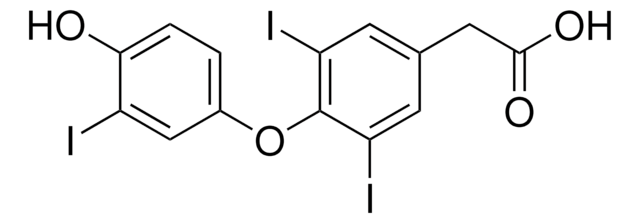
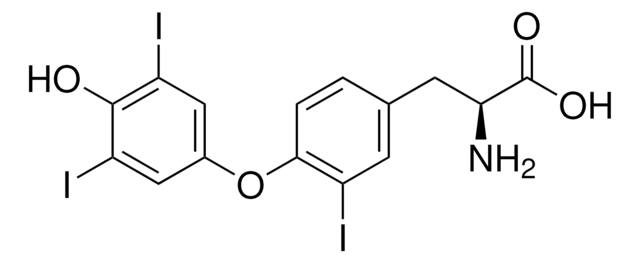
3,3′,5,5′-Tetraiodothyroacetic acid
≥98% (TLC), powder, thyrointegrin receptor antagonist
Synonym(s):
4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodobenzeneacetic acid, Tetrac
About This Item
Recommended Products
Product Name
3,3′,5,5′-Tetraiodothyroacetic acid,
solubility
acetone: soluble 19.60-20.40 mg/mL, clear, colorless (or faintly yellow to yellow)
Quality Level
storage temp.
−20°C
SMILES string
OC(=O)Cc1cc(I)c(Oc2cc(I)c(O)c(I)c2)c(I)c1
InChI
1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20)
InChI key
PPJYSSNKSXAVDB-UHFFFAOYSA-N
Application
- as a positive control to study the effects of ioxynil (IOX)
- diethylstilbestrol (DES) exposure on zebrafish embryos
- to study its effects on long-term potentiation (LTP) and long-term depression (LTD) in the dentate gyrus in urethane-anesthetized male rats
- to determine its influence on the actions of thyroid-stimulating hormone
- thyroid-stimulating immunoglobulins in orbital fibroblast
Biochem/physiol Actions
Linkage
Preparation Note
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service