SML1791
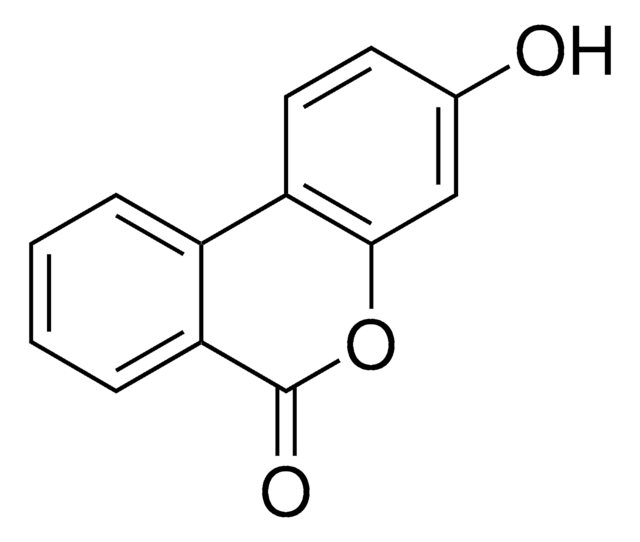
Urolithin A
≥97% (HPLC), powder, anti-inflammatory
Synonym(s):
3,8-Dihydroxy-6H-benzo[c]chromen-6-one
About This Item
Recommended Products
Product Name
Urolithin A, ≥97% (HPLC)
Quality Level
Assay
≥97% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 20 mg/mL, clear
storage temp.
2-8°C
SMILES string
OC1=CC=C(C(C=CC(O)=C2)=C2C(O3)=O)C3=C1
InChI
1S/C13H8O4/c14-7-1-3-9-10-4-2-8(15)6-12(10)17-13(16)11(9)5-7/h1-6,14-15H
InChI key
RIUPLDUFZCXCHM-UHFFFAOYSA-N
Application
- to test its antileukemic activities on leukemic cell lines by in vitro cell proliferation assay
- as a mitophagy activator to study the effect of phosphoglycerate mutase 5 (PGAM5) expression on mitophagic cell death during ischemia-reperfusion (I/R) injury
- to study its inhibitory effect on epithelial-to-mesenchymal transition (EMT) in lung cancer cells via P53-Mdm2-Snail pathway
- to study its effects on streptozotocin-induced diabetic cardiomyopathy in rats by activating sirtuin 1/silent mating type information regulation 2 homolog 1 (SIRT1) signaling
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service