S4264
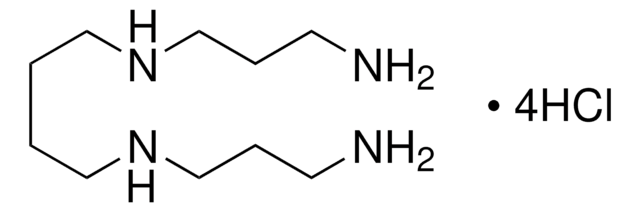
Spermine
suitable for cell culture, BioReagent
Synonym(s):
N,N′-Bis(3-aminopropyl)-1,4-diaminobutane, Gerontine, Musculamine, Neuridine
About This Item
Recommended Products
biological source
microbial
synthetic
product line
BioReagent
Assay
≥96%
form
solid
technique(s)
cell culture | mammalian: suitable
bp
150 °C/5 mmHg (lit.)
mp
28-30 (lit.)
solubility
H2O: 50 mg/mL
storage temp.
2-8°C
SMILES string
[H]N(CCCN)CCCCN([H])CCCN
InChI
1S/C10H26N4/c11-5-3-9-13-7-1-2-8-14-10-4-6-12/h13-14H,1-12H2
InChI key
PFNFFQXMRSDOHW-UHFFFAOYSA-N
Gene Information
human ... GRIN2B(2904)
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Application
- in the buffer, to swap the divalent cations in the MgSO4 protocol to stabilize the integrity of the chromosomes
- in the preparation of modified mica surfaces from freshly cleaved mica surfaces
- to study its effects on survival in mice with sepsis and to estimate the role of spermine in lethal systemic inflammatory diseases
Biochem/physiol Actions
Mixed NMDA glutamate receptor agonist/antagonist at the polyamine site. Neuroprotective effects have been observed at high concentrations (1 mM), while neurotoxicity is observed at lower concentrations. It enhances agonist effectiveness at the strychnine-insensitive glycine site. Plays a role in cellular proliferation and differentiation; inhibits neuronal nitric oxide synthase (nNOS).
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service