F5016
Anti-Human IgG (Fc specific)−FITC antibody, Mouse monoclonal
clone HP-6017, purified from hybridoma cell culture
Synonym(s):
Monoclonal Anti-Human IgG (Fc specific)
About This Item
Recommended Products
biological source
mouse
conjugate
FITC conjugate
antibody form
purified from hybridoma cell culture
antibody product type
secondary antibodies
clone
HP-6017, monoclonal
form
buffered aqueous solution
species reactivity
rabbit, sheep, horse (IgG), goat, human
storage condition
protect from light
technique(s)
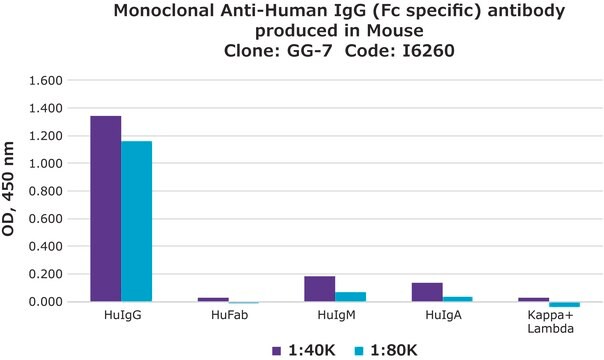
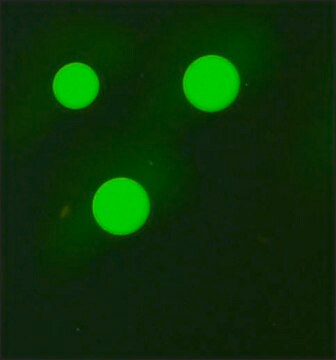
dot immunobinding: 1:16
particle immunofluorescence: 1:16
isotype
IgG2a
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Looking for similar products? Visit Product Comparison Guide
Related Categories
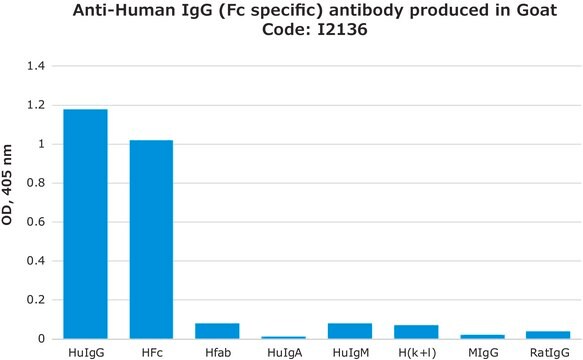
General description
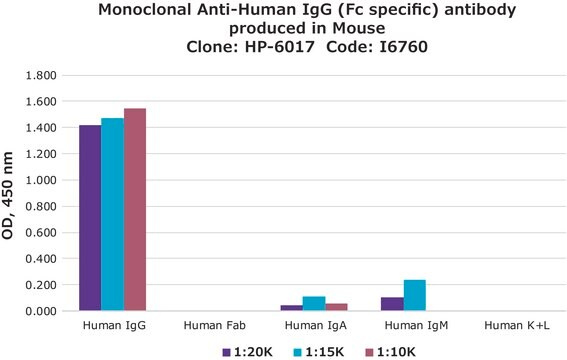
Specificity
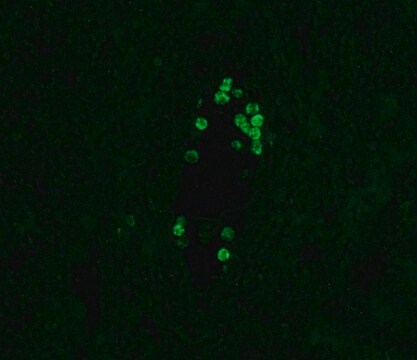
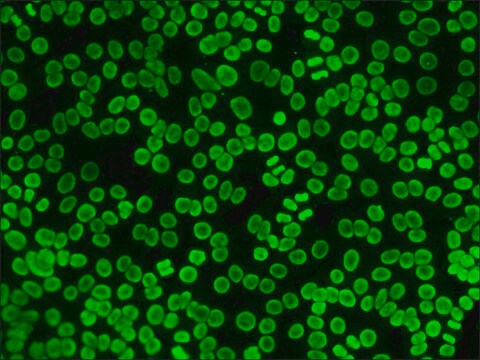
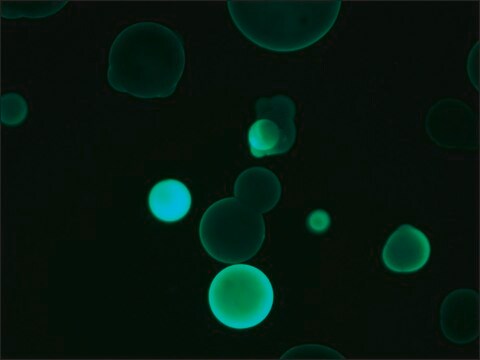
Application
- Fluorescent Dot Immunobinding Assay (F-DIBA)
- Particle Immunofluorescent Assay (F-IFMA)
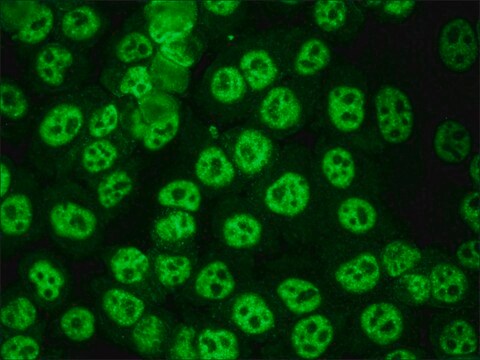
- flow cytometry
Biochem/physiol Actions
Physical form
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service