D9761
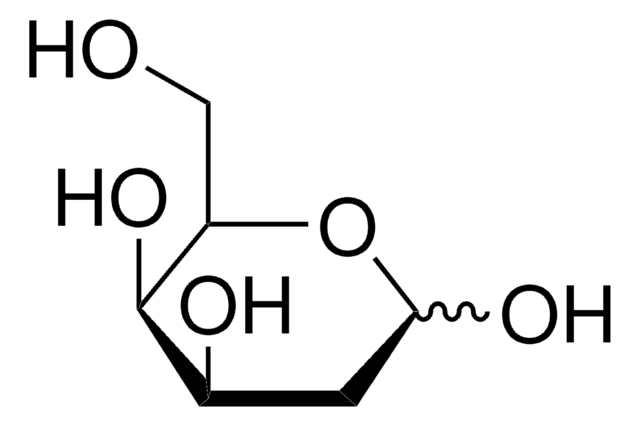
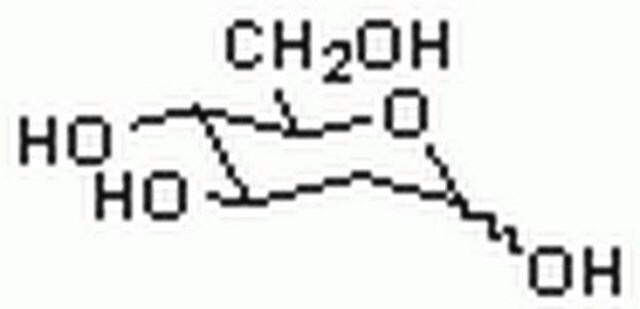
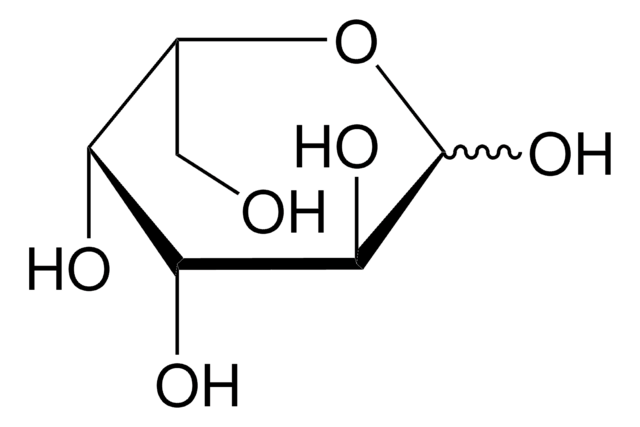
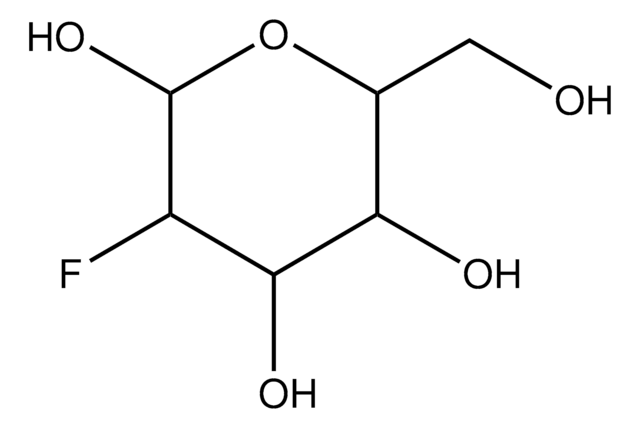
6-Deoxy-D-glucose
Synonym(s):
D-Isorhamnose, Epifucose, Quinovose
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H12O5
CAS Number:
Molecular Weight:
164.16
Beilstein:
1723317
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.52
Recommended Products
grade
Molecular Biology
for molecular biology
Quality Level
Assay
≥98% (TLC)
form
powder
storage temp.
−20°C
SMILES string
C[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H12O5/c1-2-3(7)4(8)5(9)6(10)11-2/h2-10H,1H3/t2-,3-,4+,5-,6?/m1/s1
InChI key
SHZGCJCMOBCMKK-GASJEMHNSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Deoxy-D-glucose is a structural homolog of D-glucose (dextrose) and stable analog. It lacks the hydroxyl group at carbon 6 position. It is also an analog of mannose.
Application
6-Deoxy-D-glucose has been used as a standard in the circular dichroism measurements. It has also been used as sugar to incubate starved Dictyostelium HMX44A.atg1-1 cells for microscopy studies.
Biochem/physiol Actions
2-Deoxy-D-glucose (2-DG) is used as a glycolytic inhibitor in studying the biological function of glucose. It is not metabolized, induces endoplasmic reticulum stress and hence, blocks the carbohydrate metabolism in cancer cells. It has therapeutic potential in targeting chemo-resistant hypoxic cancer cells. 2-DG halts the N-linked glycosylation by replacing mannose.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D Granot et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(13), 5724-5728 (1991-07-01)
Nutrients play a critical role in the decision to initiate a new cell cycle. Addition of nutrients to arrested cells such as stationary-phase cells and spores induces them to begin growth. We have analyzed the nutrients required to induce early
A H Romano
Journal of bacteriology, 152(3), 1295-1297 (1982-12-01)
6-Deoxy-D-glucose, a structural homomorph of D-glucose which lacks a hydroxyl group at carbon 6 and thus cannot be phosphorylated, is transported by Saccharomyces cerevisiae via a facilitated diffusion system with affinity equivalent to that shown with D-glucose. This finding supports
C Laporte et al.
Cell death and differentiation, 14(2), 266-274 (2006-07-01)
While necrotic cell death is attracting considerable interest, its molecular bases are still poorly understood. Investigations in simple biological models, taken for instance outside the animal kingdom, may benefit from less interference from other cell death mechanisms and from better
Cristina De Castro et al.
Glycobiology, 23(3), 346-353 (2012-10-19)
A major virulence factor for Yersinia pseudotuberculosis is lipopolysaccharide, including O-polysaccharide (OPS). Currently, the OPS based serotyping scheme for Y. pseudotuberculosis includes 21 known O-serotypes, with genetic and structural data available for 17 of them. The completion of the OPS
A J Fry et al.
Molecular and biochemical parasitology, 60(1), 9-18 (1993-07-01)
Kinetic parameters for entry of D-fructose into Trypanosoma brucei brucei have been determined. The net uptake of D-fructose was found to be rapid and occurred at a rate which was comparable with that observed for uptake of D-glucose. The Km
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service