D9305
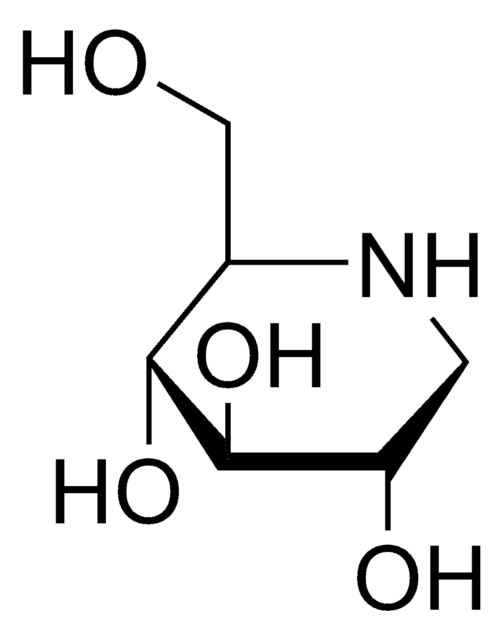
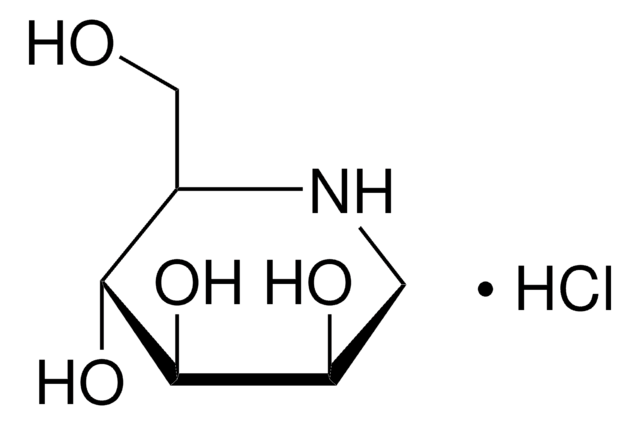
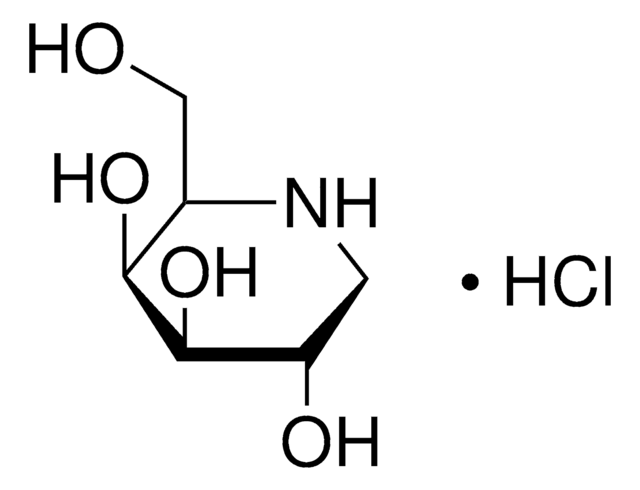
1-Deoxynojirimycin hydrochloride
Synonym(s):
1,5-Dideoxy-1,5-imino-D-sorbitol hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H13NO4 · HCl
CAS Number:
Molecular Weight:
199.63
Beilstein:
3563225
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
≥98% (TLC)
Quality Level
form
powder
solubility
water: 19.60-20.40 mg/mL, clear, colorless
antibiotic activity spectrum
viruses
Mode of action
enzyme | inhibits
storage temp.
2-8°C
SMILES string
Cl[H].OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13NO4.ClH/c8-2-3-5(10)6(11)4(9)1-7-3;/h3-11H,1-2H2;1H/t3-,4+,5-,6-;/m1./s1
InChI key
ZJIHMALTJRDNQI-VFQQELCFSA-N
Gene Information
human ... GAA(2548)
Looking for similar products? Visit Product Comparison Guide
General description
Chemical structure: glucosamine
Application
1-Deoxynojirimycin hydrochloride has been used
- to study its effects on the loss-of-function of N-glycosylation pathway on hair cell regeneration
- as an endoplasmic reticulum (ER) α-glucosidase I and II inhibitor to study its effects on TMED3-cystic fibrosis transmembrane conductance regulator (CFTR) interaction
- as an insect trehalase inhibitor in TREH inhibition bioassay
Biochem/physiol Actions
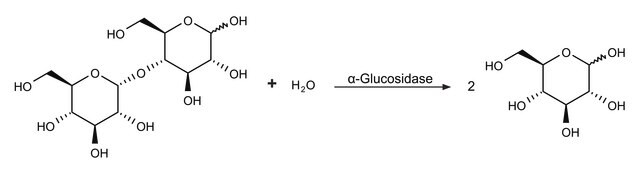
1-Deoxynojirimycin (DNJ) is a naturally occurring alkaloid azasugar or iminosugar. It is observed in mulberry leaves, Commelina communis, and bacterial strains including Bacillus and Streptomyces species. DNJ acts as an α-glucosidase inhibitor and exhibits anti-viral anti-diabetic, anti-inflammatory, anti-obesity, and antioxidant properties.
α-Glucosidase inhibitor
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marc C Patterson et al.
Orphanet journal of rare diseases, 8, 12-12 (2013-01-18)
Niemann-Pick disease type C (NP-C) is a rare neurovisceral disease characterized by progressive neurodegeneration and premature death. We report data recorded at enrolment in an ongoing international NP-C registry initiated in September 2009 to describe disease natural history, clinical course
Timothy M Cox et al.
Orphanet journal of rare diseases, 7, 102-102 (2012-12-29)
Previous studies have provided equivocal data on the use of miglustat as maintenance therapy in Gaucher disease type 1. We report findings from a clinical trial evaluating the effects of miglustat treatment in patients with stable type 1 Gaucher disease
Stephen G Davies et al.
Organic letters, 15(8), 2042-2045 (2013-04-11)
The asymmetric syntheses of (-)-1-deoxymannojirimycin and (+)-1-deoxyallonojirimycin are described herein. The ring-closing iodoamination of two epimeric bishomoallylic amines to give the corresponding 5-iodomethylpyrrolidines was followed by in situ ring-expansion to give two diastereoisomerically pure (>99:1 dr) cyclic carbonates. Subsequent deprotection
Chaluntorn Vichasilp et al.
Food chemistry, 134(4), 1823-1830 (2013-02-28)
Mulberry 1-deoxynojirimycin (DNJ), a potent α-glycosidase inhibitor, has therapeutic potency in the suppression of postprandial blood glucose levels thereby possibly preventing diabetes mellitus. However, DNJ has a relatively short half-life in vivo (about 2 h). Therefore, several doses of mulberry
Hee-Woong Kim et al.
Microorganisms, 11(6) (2023-06-28)
This study examines the possibility of directly producing and utilizing useful substances in the intestines of animals using anaerobic bacteria that can grow in the intestines of animals. A facultative anaerobe producing a large amount of α-glucosidase inhibitor was isolated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service