C3506
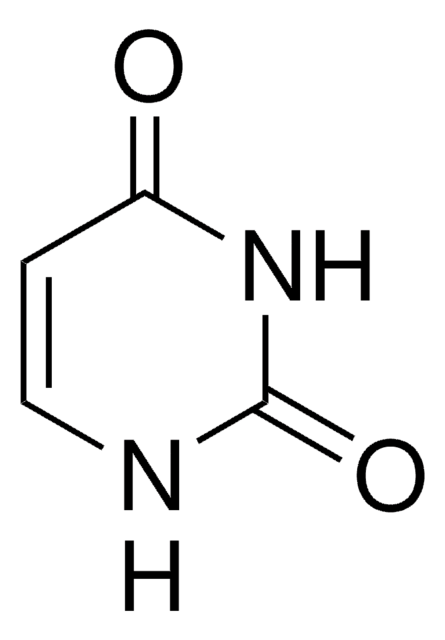
Cytosine
≥99%
Synonym(s):
4-Amino-2-hydroxypyrimidine, 4-Aminopyrimidin-2-(1H)-one
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C4H5N3O
CAS Number:
Molecular Weight:
111.10
Beilstein:
2637
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥99%
form
powder
mp
>300 °C (lit.)
solubility
0.5 M HCl: 50 mg/mL, clear to very slightly hazy, colorless to faintly yellow
SMILES string
NC1=NC(=O)NC=C1
InChI
1S/C4H5N3O/c5-3-1-2-6-4(8)7-3/h1-2H,(H3,5,6,7,8)
InChI key
OPTASPLRGRRNAP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cytosine has been used:
- for the preparation of nucleobase solutions
- as a standard for high-performance liquid chromatography (HPLC)
- for the estimation of global methylation rate
- for nucleoside 5′-triphosphate (NTP) synthesis
- purification
Biochem/physiol Actions
Cytosine (C) is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA).
Cytosine is a pyrimidine, which forms three hydrogen bonds to base pair with guanine. It forms a nucleotide cytidine, that is phosphorylated to cytidine 5′ monophosphate (CMP), cytidine 5′ diphosphate (CDP) and cytidine 5′ triphosphate (CTP).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Encyclopedia of Genetics (2001)
Deuterated nucleotides as chemical probes of RNA structure: a detailed protocol for the enzymatic synthesis of a complete set of nucleotides specifically deuterated at ribose carbons
Azad R, et al.
ScienceOpen Research (2015)
Akira Ono et al.
Chemical Society reviews, 40(12), 5855-5866 (2011-08-10)
Pyrimidine base pairs in DNA duplexes selectively capture metal ions to form metal ion-mediated base pairs, which can be evaluated by thermal denaturation, isothermal titration calorimetry, and nuclear magnetic resonance spectroscopy. In this critical review, we discuss the metal ion
Nucleobase sensing using highly-sensitive surface-enhanced Raman spectroscopy templates comprising organic semiconductor peptide nanotubes and metal nanoparticles
Almohammed S, et al.
Sensing and Bio-Sensing Research, 24, 100287-100287 (2019)
Transition from somatic embryo to friable embryogenic callus in cassava: dynamic changes in cellular structure, physiological status, and gene expression profiles
Ma Q, et al.
Frontiers in Plant Science, 6, 824-824 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service