C3389
Acetylcholinesterase from Electrophorus electricus (electric eel)
Type VI-S, lyophilized powder, 200-1,000 units/mg protein
Synonym(s):
AChE, Acetylcholine acetylhydrolase, Cholinesterase, Acetyl
Sign Into View Organizational & Contract Pricing
All Photos(7)
About This Item
Recommended Products
type
Type VI-S
Quality Level
form
lyophilized powder
specific activity
200-1,000 units/mg protein
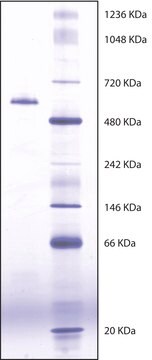
mol wt
280 kDa
composition
Protein, ≥45% biuret
solubility
20 mM Tris HCl buffer, pH 7.5: soluble 1.0 mg/mL, clear(lit.)
application(s)
diagnostic assay manufacturing
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Molecular Weight: 280 kDa
Isoelectric Point: 5.5
Extinction Coefficient: E1% = 18.0 (280 nm)
Acetylcholinesterase from Electrophorus electricus is a tetramer composed of 4 equal subunits of 70 kDa each. Each subunit contains one active site. The enzyme is a glycoprotein containing hexosamines.
Isoelectric Point: 5.5
Extinction Coefficient: E1% = 18.0 (280 nm)
Acetylcholinesterase from Electrophorus electricus is a tetramer composed of 4 equal subunits of 70 kDa each. Each subunit contains one active site. The enzyme is a glycoprotein containing hexosamines.
Application
The enzyme from sigma has been used as a reference to to evaluate the effect of aspartame metabolites on hippocampal acetylcholinesterase activity. The enzyme has also been used in immobilization studies for the rapid detection of acetylthiocholine chloride.
Biochem/physiol Actions
Major degradative enzyme for acetylcholine in vivo. Converts acetylcholine + H2O to choline + acetic acid.
Unit Definition
One unit will hydrolyze 1.0 μmole of acetylcholine to choline and acetate per min at pH 8.0 at 37 °C.
Physical form
Lyophilized powder containing Tris buffer salts
Preparation Note
This enzyme dissolves in 20 mM Tris-HCl buffer, pH 7.5 at 1 mg/mL concentration, yielding a clear solution.
Analysis Note
The activity obtained using acetylcholine as substrate is 30-100 times that obtained with butyrylcholine, using acetylcholinesterase from electric eel.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dan Du et al.
Analytical and bioanalytical chemistry, 387(3), 1059-1065 (2006-12-23)
A simple method has been devised for immobilization of acetylcholinesterase (AChE)--covalent bonding to a multiwall carbon nanotube (MWNT)--cross-linked chitosan composite (CMC)-and a sensitive amperometric sensor for rapid detection of acetylthiocholine (ATCl) has been based on this. Fourier-transform infrared spectroscopy proved
Jingming Gong et al.
Biosensors & bioelectronics, 24(7), 2285-2288 (2008-12-30)
We developed a simple strategy for designing a highly sensitive electrochemical biosensor for organophosphate pesticides (OPs) based on acetylcholinesterase (AChE) immobilized onto Au nanoparticles-polypyrrole nanowires composite film modifid glassy carbon electrode (labeled as AChE-Au-PPy/GCE). Where, the generated Au nanoparticles (AuNPs)
Gabriela Villalta et al.
Plants (Basel, Switzerland), 10(6) (2021-07-03)
The essential oil (EO) of Salvia leucantha Cav. was isolated by steam distillation of the aerial parts collected in the South of Ecuador. Its physical properties were evaluated and the chemical composition of the oil was determined by GC-MS and
Makar Makarian et al.
Journal of molecular structure, 1247 (2022-03-01)
In an effort to develop new therapeutic agents to treat Alzheimer's disease, a series of donepezil-based analogs were designed, synthesized using an environmentally friendly route, and biologically evaluated for their inhibitory activity against electric eel acetylcholinesterase (AChE) enzyme. In vitro
Acetylcholinesterase, I. Large-scale purification, homogeneity, and amino Acid analysis.
W Leuzinger et al.
Proceedings of the National Academy of Sciences of the United States of America, 57(2), 446-451 (1967-02-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service