A3835
Ascomycin from Streptomyces hygroscopicus var. ascomyceticus
Synonym(s):
Changchuanmycin, FK-520, FR900520, Immunomycin, L 683590
About This Item
Recommended Products
form
powder
Quality Level
greener alternative product score
old score: 59
new score: 54
Find out more about DOZN™ Scoring
greener alternative product characteristics
Designing Safer Chemicals
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
antibiotic activity spectrum
fungi
greener alternative category
Mode of action
enzyme | inhibits
storage temp.
−20°C
SMILES string
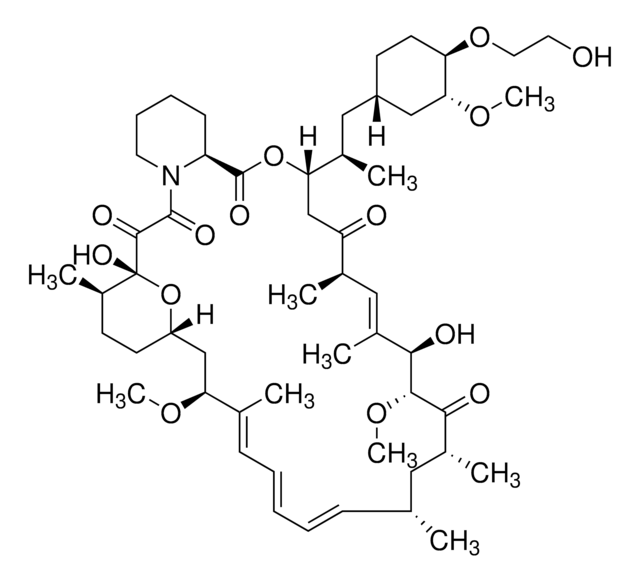
CCC1\C=C(C)\C[C@H](C)C[C@H](OC)C2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N3CCCC[C@H]3C(=O)OC([C@H](C)[C@@H](O)CC1=O)\C(C)=C\[C@@H]4CC[C@@H](O)[C@@H](C4)OC
InChI
1S/C43H69NO12/c1-10-30-18-24(2)17-25(3)19-36(53-8)39-37(54-9)21-27(5)43(51,56-39)40(48)41(49)44-16-12-11-13-31(44)42(50)55-38(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)52-7/h18,20,25,27-33,35-39,45-46,51H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31-,32+,33-,35+,36-,37-,38+,39+,43+/m0/s1
InChI key
ZDQSOHOQTUFQEM-NURRSENYSA-N
General description
Biochem/physiol Actions
Linkage
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service