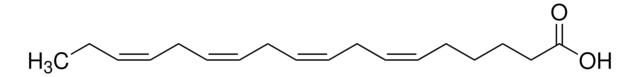
A3611
Arachidonic acid
from non-animal source, ≥98.5% (GC)
Synonym(s):
cis,cis,cis,cis-5,8,11,14-Eicosatetraenoic acid, Eicosa-5Z,8Z,11Z,14Z-tetraenoic acid, Immunocytophyte
About This Item
Recommended Products
biological source
non-animal source
Quality Level
Assay
≥98.5% (GC)
form
liquid
refractive index
n20/D 1.4872 (lit.)
bp
169-171 °C/0.15 mmHg (lit.)
mp
−49 °C (lit.)
density
0.922 g/mL at 25 °C (lit.)
functional group
carboxylic acid
lipid type
omega FAs
shipped in
dry ice
storage temp.
−20°C
SMILES string
OC(CCC/C=C\C/C=C\C/C=C\C/C=C\CCCCC)=O
InChI
1S/C20H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12-,16-15-
InChI key
YZXBAPSDXZZRGB-DOFZRALJSA-N
Looking for similar products? Visit Product Comparison Guide
Application
<li><strong>Molecular Mechanisms Associated with the Inhibitory Role of Long Chain n-3 PUFA in Colorectal Cancer:</strong> This study discusses the effects of long-chain polyunsaturated fatty acids, like arachidonic acid, on colorectal cancer mechanisms. The research focuses on the anti-inflammatory and cancer inhibitory roles through the modulation of lipid metabolism and signal transduction pathways (Jayathilake et al., 2024).</li>
<li><strong>Zhilining Formula alleviates DSS-induced colitis through suppressing inflammation and gut barrier dysfunction via the AHR/NF-Bp65 axis:</strong> This article presents arachidonic acid′s role in the suppression of inflammation and restoration of gut barrier function, crucial for understanding inflammatory diseases and developing therapeutic strategies (Zhou et al., 2024).</li>
<li><strong>5,6-diHETE lactone (EPA-L) mediates hypertensive microvascular dilation by activating the endothelial GPR-PLC-IP(3) signaling pathway:</strong> Explores the cardiovascular implications of arachidonic acid metabolites, specifically their role in microvascular responses, which could influence hypertension treatment strategies (Asulin et al., 2024).</li>
</ul>
Biochem/physiol Actions
Packaging
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service