A3381
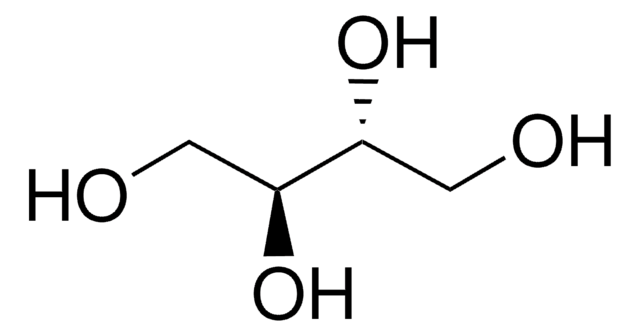
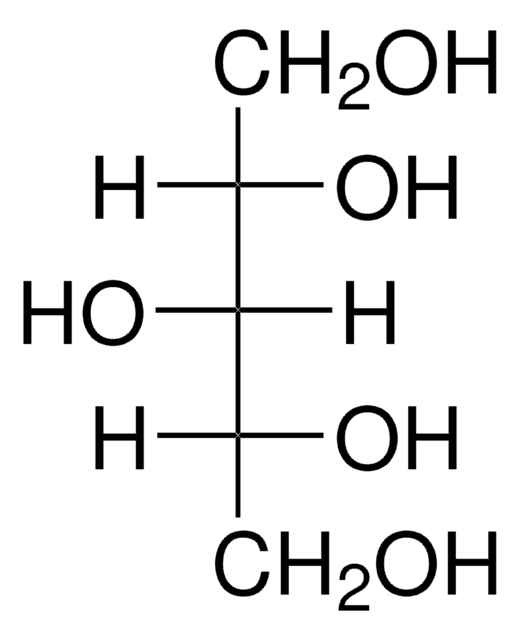
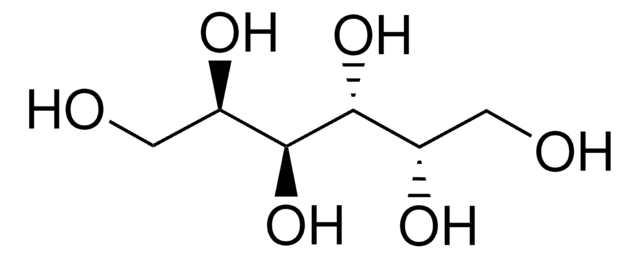
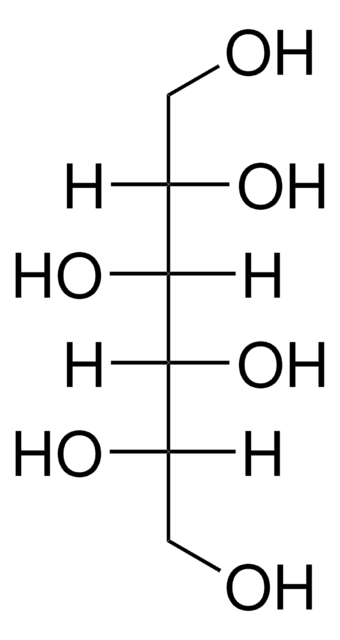
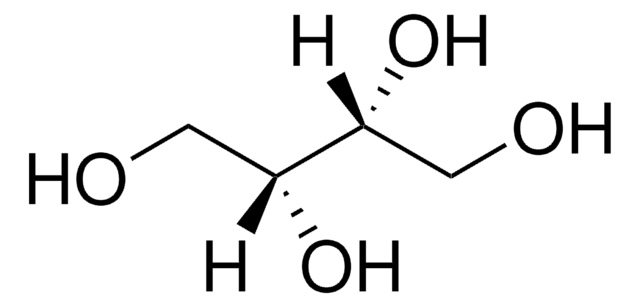
D-(+)-Arabitol
≥99% (GC)
Synonym(s):
D-arabino-Pentitol, D-lyxo-Pentitol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H12O5
CAS Number:
Molecular Weight:
152.15
Beilstein:
1720520
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥99% (GC)
form
powder
sweetness
0.7 × sucrose
color
white
solubility
water: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
room temp
SMILES string
OC[C@@H](O)C(O)[C@H](O)CO
InChI
1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2/t3-,4-/m1/s1
InChI key
HEBKCHPVOIAQTA-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
Application
D-Arabitol, a rare sugar alcohol, is a substrate used to identify, differentiate and characterize enzyme such as the gluconobacter oxydans dehydrogenase(s), Gox2181, hyperthermophilic D-arabitol dehydrogenase from Thermotoga maritime and NAD-dependent D-arabitoldehydrogenase from acetic acid bacterium, Acetobacter suboxydans.
Biochem/physiol Actions
Used to produce xylitol via biotransformation.
Other Notes
To gain a comprehensive understanding of our extensive range of Sugar alcohols for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Verena Kallnik et al.
Applied microbiology and biotechnology, 90(4), 1285-1293 (2011-02-25)
The first hyperthermophilic D-arabitol dehydrogenase from Thermotoga maritima was heterologously purified from Escherichia coli. The protein was purified with and without a Strep-tag. The enzyme exclusively catalyzed the NAD(H)-dependent oxidoreduction of D-arabitol, D-xylitol, D-ribulose, or D-xylulose. A twofold increase of
Vivien Krell et al.
World journal of microbiology & biotechnology, 34(8), 108-108 (2018-07-05)
Successful commercialization of microbial biocontrol agents, such as Metarhizium spp., is often constrained by poor drying survival and shelf life. Here, we hypothesized that culture age would influence endogenous arabitol, erythritol, mannitol and trehalose contents in M. brunneum mycelium and
Xu Liu et al.
Biochemical and biophysical research communications, 415(2), 410-415 (2011-11-02)
Gluconobacter oxydans enable to oxidize sugars and polyols incompletely to corresponding materials with potential industrial applications, containing around 75 putative dehydrogenases. One of these putative dehydrogenases, Gox2181, was cloned and expressed in Escherichia coli BL21 (DE3), and its X-ray crystal
L Schakenraad et al.
Placenta, 104, 220-231 (2021-01-12)
An increasing number of women becomes pregnant while suffering from chronic kidney disease (CKD). As a result of decreased renal function, uremic solutes circulate at high levels in the maternal circulation. This study aimed to acquire more knowledge about the
High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio.
Zhiguo Zhang et al.
International journal of molecular sciences, 15(9), 17130-17147 (2014-09-27)
The aim of this study was to evaluate effect of diosgenin (DG) on rats that had osteoporosis-like features induced by ovariectomy (OVX). Seventy-two six-month-old female Wistar rats were subjected to either ovariectomy (n = 60) or Sham operation (SHAM group
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service