41659
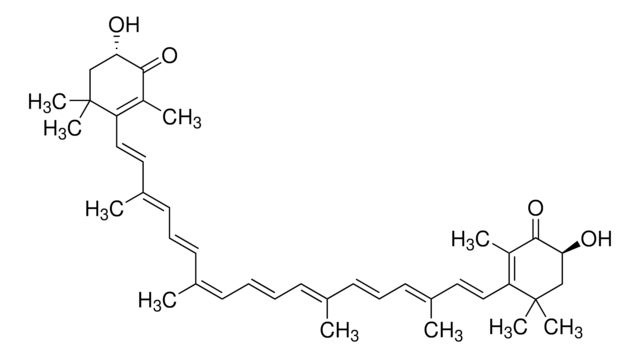
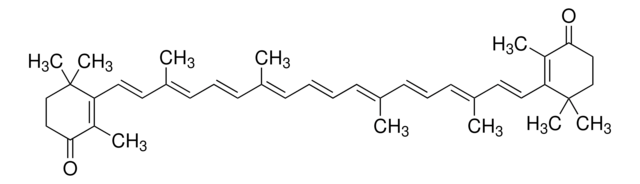
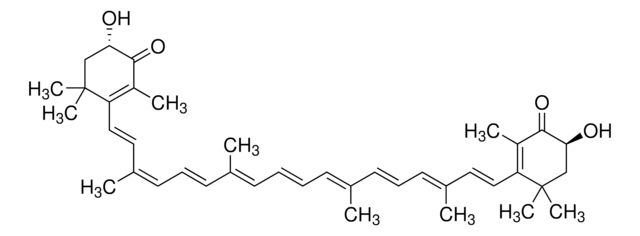
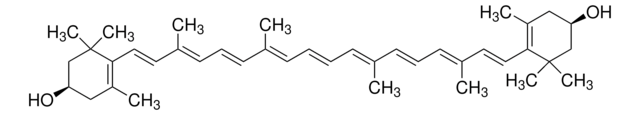
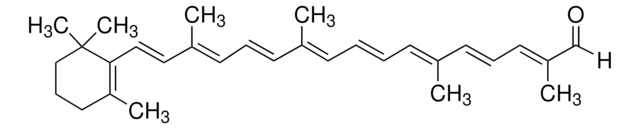
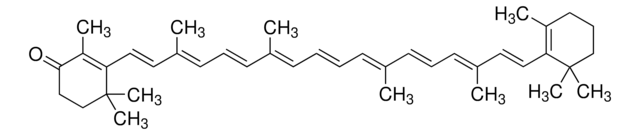
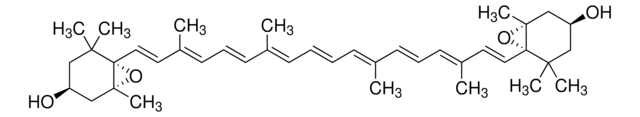
all-trans-Astaxanthin
analytical standard
Synonym(s):
Astaxanthin, (3S,3′S,all-trans)-3,3′-Dihydroxy-β,β-carotene-4,4′-dione, (3S,3′S)-3,3′-Dihydroxy-β,β-carotene-4,4′-dione
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥97%
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
storage temp.
−20°C
SMILES string
CC1=C(/C=C/C(C)=C/C=C/C(C)=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C2=C(C)C([C@@H](O)CC2(C)C)=O)C(C)(C)C[C@H](O)C1=O
InChI
1S/C40H52O4/c1-27(17-13-19-29(3)21-23-33-31(5)37(43)35(41)25-39(33,7)8)15-11-12-16-28(2)18-14-20-30(4)22-24-34-32(6)38(44)36(42)26-40(34,9)10/h11-24,35-36,41-42H,25-26H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,24-22+,27-15+,28-16+,29-19+,30-20+/t35-,36-/m0/s1
InChI key
MQZIGYBFDRPAKN-UWFIBFSHSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Microalgae (Green algae), Haematococcus pluvialis using high-performance liquid chromatography (HPLC) with mass spectrometric and fluorescence detection (HPLC-FLD).
- Parapenaeopsis hardwickii using high-performance liquid chromatography (HPLC) with photodiode array detection (PDA).
Biochem/physiol Actions
Packaging
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
HPLC Analysis of Carotene Compounds on Ascentis® RP-Amide
Related Content
Separation of Astaxanthin; Xanthophyll
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service