All Photos(3)
About This Item
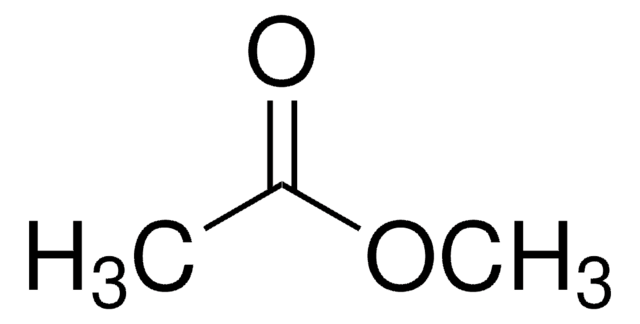
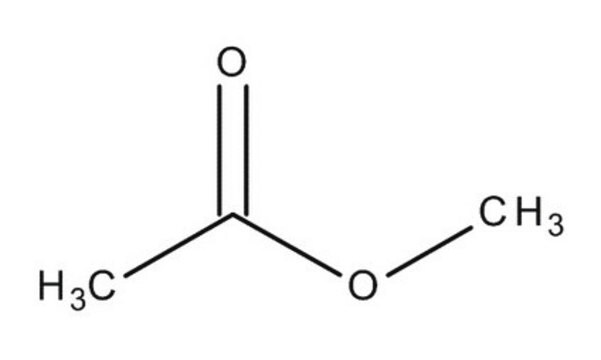
Linear Formula:
CH3COOCH3
CAS Number:
Molecular Weight:
74.08
Beilstein:
1736662
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
anhydrous
Quality Level
vapor density
2.55 (vs air)
vapor pressure
165 mmHg ( 20 °C)
Assay
99.5%
form
liquid
autoignition temp.
936 °F
expl. lim.
16 %
impurities
<0.003% water
<0.005% water (100 mL pkg)
evapn. residue
<0.0003%
refractive index
n20/D 1.361 (lit.)
bp
57-58 °C (lit.)
mp
−98 °C (lit.)
density
0.934 g/mL at 25 °C
SMILES string
COC(C)=O
InChI
1S/C3H6O2/c1-3(4)5-2/h1-2H3
InChI key
KXKVLQRXCPHEJC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl acetate (MA) is an aliphatic ester that can be prepared via carbonylation of dimethyl ether over zeolites. MA is formed as a by-product during the preparation of polyvinyl alcohol from acetic acid and methanol.
Application
Methyl acetate may be used for the preparation of fatty acid methyl esters and triacetin from rapeseed oil via non-catalytic trans-esterification reaction under super-critical conditions.
Other Notes
The article number 296996-6X1L will be discontinued. Please order the single bottle 296996-1L which is physically identical with the same exact specifications.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
8.6 °F - closed cup
Flash Point(C)
-13 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reaction kinetics and chemical equilibrium of homogeneously and heterogeneously catalyzed acetic acid esterification with methanol and methyl acetate hydrolysis
Popken T, et al.
Industrial & Engineering Chemistry Research, 39(7), 2601-2611 (2000)
Kinetics of transesterification of methyl acetate and n-octanol catalyzed by cation exchange resins.
Liu Y, et al.
Korean Journal of Chemical Engineering, 30(5), 1039-1042 (2013)
Catalysts, Kinetics, and Reactive Distillation for Methyl Acetate Synthesis.
Zuo C, et al.
Industrial & Engineering Chemistry Research, 53(26), 10540-10548 (2014)
A new process for catalyst-free production of biodiesel using supercritical methyl acetate.
Saka S and Isayama Y.
Fuel: The Science and Technology of Fuel and Energy, 88(7), 1307-1313 (2009)
Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites.
Patricia Cheung et al.
Angewandte Chemie (International ed. in English), 45(10), 1617-1620 (2006-01-31)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service