104043
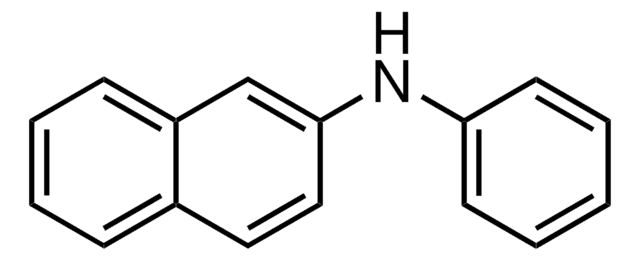
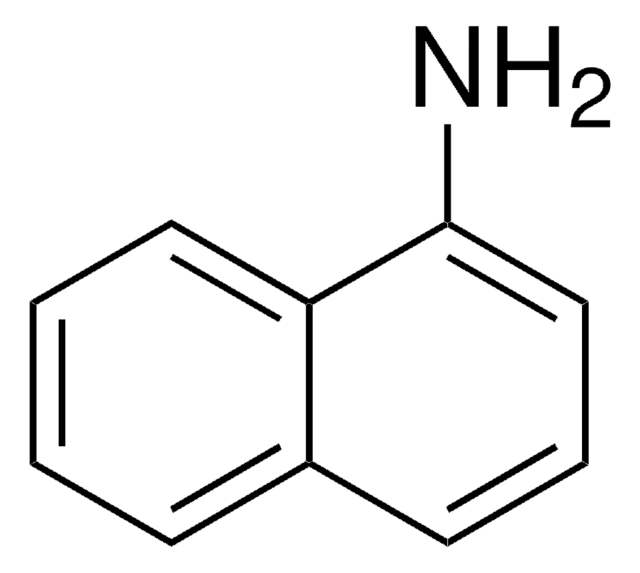
N-Phenyl-1-naphthylamine
reagent grade, 98%
Synonym(s):
1-(N-phenylamino)naphthalene, N-(1-Naphthyl)aniline, NPN
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H7NHC6H5
CAS Number:
Molecular Weight:
219.28
Beilstein:
2211174
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
reagent grade
Quality Level
Assay
98%
form
solid
bp
226 °C/15 mmHg (lit.)
mp
60-62 °C (lit.)
λmax
252 nm
SMILES string
N(c1ccccc1)c2cccc3ccccc23
InChI
1S/C16H13N/c1-2-9-14(10-3-1)17-16-12-6-8-13-7-4-5-11-15(13)16/h1-12,17H
InChI key
XQVWYOYUZDUNRW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Phenyl-1-naphthylamine can be used as fluorescent probe for the determination of critical micelle concentration of surfactants. N-Phenyl-1-naphthylamine was used in a method for determination of the concentration of organolithium and organomagnesium reagents. N-Phenyl-1-naphthylamine was used as hydrophobic probe to study the phase transitions of membrane lipids in whole cells .
Biochem/physiol Actions
N-Phenyl-1-naphthylamine turns fluorescent after binding to hydrophobic regions of cell membranes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1B - STOT RE 2
Target Organs
Blood
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiaoyan Ning et al.
International journal of molecular sciences, 19(2) (2018-02-01)
Glucose oxidase (GOD, EC.1.1.3.4) specifically catalyzes the reaction of β-d-glucose to gluconic acid and hydrogen peroxide in the presence of oxygen, which has become widely used in the food industry, gluconic acid production and the feed industry. However, the poor
G M Halliday et al.
Journal of immunological methods, 28(3-4), 381-390 (1979-01-01)
N-phenyl-1-naphthylamine (NPN) becomes fluorescent after binding to hydrophobic regions of cell membranes. Rat and mouse lymphoid cell suspensions stained with NPN showed changes in fluorescence emission 30 min after stimulation with mitogen or antigen, detected by microfluorimetry. Incubation of NPN-labelled
Determination of the critical micelle concentration of surfactants using the fluorescent probe N-phenyl-1-naphthylamine.
Bergbreiter DE and Pendergrass E.
The Journal of Organic Chemistry, 46(1), 219-220 (1981)
Daniel Pletzer et al.
PLoS pathogens, 14(6), e1007084-e1007084 (2018-06-22)
With the antibiotic development pipeline running dry, many fear that we might soon run out of treatment options. High-density infections are particularly difficult to treat due to their adaptive multidrug-resistance and currently there are no therapies that adequately address this
Sasitorn Chusri et al.
The Journal of antimicrobial chemotherapy, 64(6), 1203-1211 (2009-10-29)
The emergence of antibiotic resistance has seriously diminished antibiotic efficacy and an increasing number of infections are becoming difficult to treat. One approach to the restoration of antibiotic activity is to administer them in conjunction with non-antibiotic compounds that depress
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service