F16001
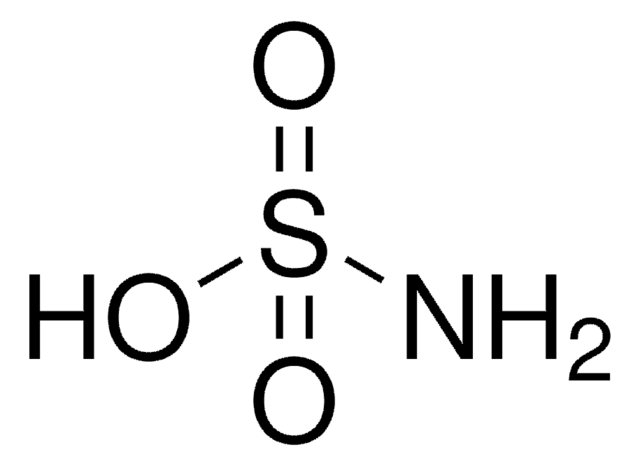
Formamidinesulfinic acid
≥98%
Synonym(s):
Aminoiminomethanesulfinic acid, Thiourea dioxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2C(=NH)SO2H
CAS Number:
Molecular Weight:
108.12
Beilstein:
506653
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
mp
124-127 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
NC(=N)S(O)=O
InChI
1S/CH4N2O2S/c2-1(3)6(4)5/h(H3,2,3)(H,4,5)
InChI key
FYOWZTWVYZOZSI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Convenient reagent for the reduction of ketones to secondary alcohols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Self-heat. 1 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Target Organs
Lungs, Respiratory system
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The use of formamidine sulphinic acid in the preparation of 99mTc-labelled radiopharmaceuticals--a cautionary note.
J Baldas et al.
International journal of nuclear medicine and biology, 8(1), 110-111 (1981-01-01)
Preparation of technetium-99m glucoheptonate utilizing formamidine sulfinic acid.
J R Scott et al.
International journal of nuclear medicine and biology, 7(1), 71-73 (1980-01-01)
J Baldas et al.
European journal of nuclear medicine, 7(4), 187-189 (1982-01-01)
The use of formamidine sulphinic acid as a reducing agent in the presence of technetium-99-pertechnetate and diethyldithiocarbamate ligand has been shown to yield a complex containing a Tc = CO bond. The carbon monoxide present in this complex originates from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service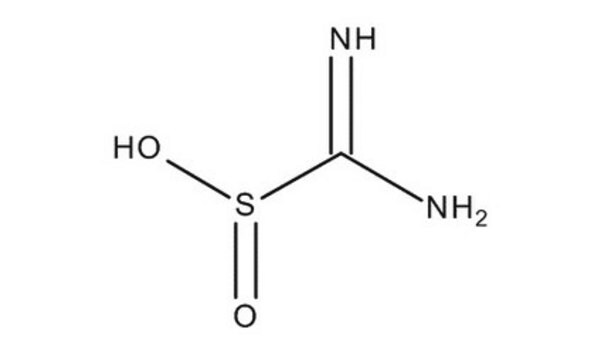


![Tris[2-(dimethylamino)ethyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/695/792/ee0ff167-22a3-43a7-83a1-6c4908adf0ae/640/ee0ff167-22a3-43a7-83a1-6c4908adf0ae.png)