E26258
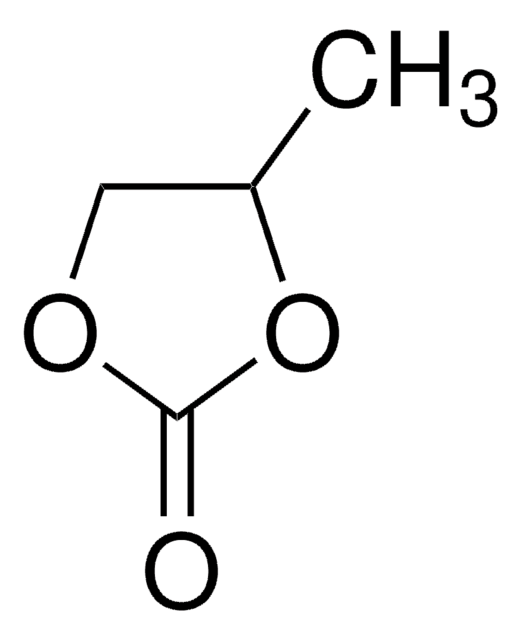
Ethylene carbonate
98%
Synonym(s):
1,3-Dioxolan-2-one
About This Item
Recommended Products
vapor density
3.04 (vs air)
vapor pressure
0.02 mmHg ( 36.4 °C)
Assay
98%
bp
243-244 °C/740 mmHg (lit.)
mp
35-38 °C (lit.)
density
1.321 g/mL at 25 °C (lit.)
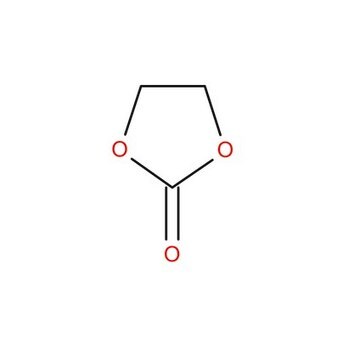
SMILES string
O=C1OCCO1
InChI
1S/C3H4O3/c4-3-5-1-2-6-3/h1-2H2
InChI key
KMTRUDSVKNLOMY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- In the synthesis of aliphatic polyurethanes using diamines and diols.
- As a precursor for ring-opening polymerization using KOH as initiator.
- As a reactant in the preparation of dimethyl carbonate, glycerol carbonate by transesterification reaction.
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 Oral
Target Organs
Kidney
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
289.4 °F - closed cup
Flash Point(C)
143 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service