B91005
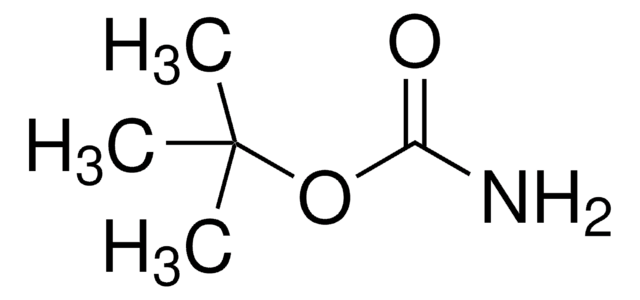
tert-Butyl carbazate
98%, for peptide synthesis
Synonym(s):
tert-Butoxycarbonyl hydrazide, Boc-hydrazide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3COCONHNH2
CAS Number:
Molecular Weight:
132.16
Beilstein:
1756967
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
tert-Butyl carbazate, 98%
Assay
98%
form
crystals
bp
63-65 °C/0.1 mmHg (lit.)
mp
39-42 °C (lit.)
application(s)
peptide synthesis
SMILES string
O=C(NN)OC(C)(C)C
InChI
1S/C5H12N2O2/c1-5(2,3)9-4(8)7-6/h6H2,1-3H3,(H,7,8)
InChI key
DKACXUFSLUYRFU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Employed in a palladium-catalyzed cross-coupling with vinyl halides leading to N-Boc-N-alkenylhydrazines.
Reagent used in solid phase peptide synthesis and in α-amino aldehyde optical purity determinations. Condenses with aldehydes to form hydrazones which are intermediates in the synthesis of HIV-1 protease inhibitors.
Reagent used in solid phase peptide synthesis and in α-amino aldehyde optical purity determinations. Condenses with aldehydes to form hydrazones which are intermediates in the synthesis of HIV-1 protease inhibitors.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
195.8 °F
Flash Point(C)
91 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 34, 5425-5425 (1993)
Journal of the Chemical Society. Chemical Communications, 1052-1052 (1993)
Tetrahedron Letters, 34, 6599-6599 (1993)
José Barluenga et al.
Organic letters, 9(2), 275-278 (2007-01-16)
N-Boc-N-alkenylhydrazines, an almost unknown type of compounds, have been prepared with high to moderate yields via palladium-catalyzed cross-coupling between alkenyl halides and tert-butyl carbazate. The present methodology represents the first general way to access this highly functionalized and unusual type
Martina Bruna Violatto et al.
ACS nano, 13(4), 4410-4423 (2019-03-19)
Steroids are the standard therapy for autoimmune hepatitis (AIH) but the long-lasting administration is hampered by severe side effects. Methods to improve the tropism of the drug toward the liver are therefore required. Among them, conjugation to nanoparticles represents one
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service