903981
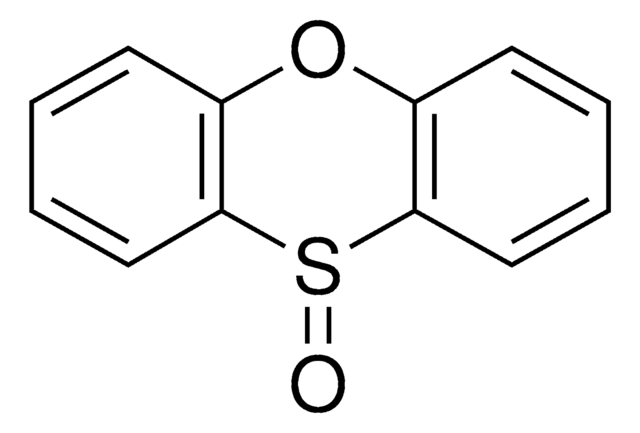
2,3,7,8-Tetrafluorothianthrene-S-oxide
≥95%
Synonym(s):
2,3,7,8-Tetrafluorothianthrene-5-oxide, Fluorinated sulfoxide-based thianthrene reagent, Ritter C-H functionalization linchpin, TFT S-oxide, TFT-reagent
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H4F4OS2
CAS Number:
Molecular Weight:
304.28
UNSPSC Code:
12352101
Recommended Products
Assay
≥95%
form
solid
mp
253 °C
storage temp.
−20°C
Related Categories
Application
2,3,7,8-Tetrafluorothianthrene-S-oxide (TFT S-oxide) is a fluorinated sulfoxide-based thianthrene reagent for late-stage C-H functionalization. Developed by the Ritter Lab, C-H functionalization by thianthrenation with TFT S-oxide proceeds with >99% selectivity and furnishes functionalized arenes serving as synthetic linchpins for further participation in diverse transformations.
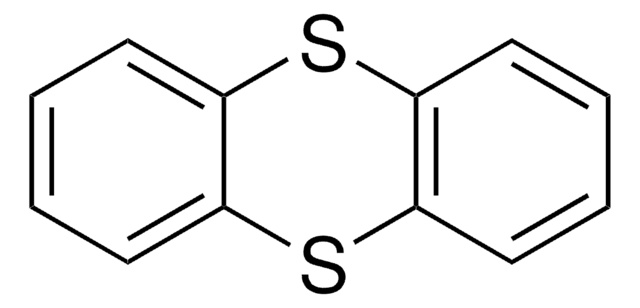
The nonfluorinated thianthrene S-oxide is also available as catalog# 903973 for all arenes more electron-rich than anisole.
The nonfluorinated thianthrene S-oxide is also available as catalog# 903973 for all arenes more electron-rich than anisole.
Other Notes
Legal Information
Patent Application EP18204755.5
This product is manufactured pursuant to a license with Studiengesellschaft Kohle mbH
This product is manufactured pursuant to a license with Studiengesellschaft Kohle mbH
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Florian Berger et al.
Nature, 567(7747), 223-228 (2019-03-15)
Direct C-H functionalization can quickly increase useful structural and functional molecular complexity1-3. Site selectivity can sometimes be achieved through appropriate directing groups or substitution patterns1-4-in the absence of such functionality, most aromatic C-H functionalization reactions provide more than one product
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)