851450
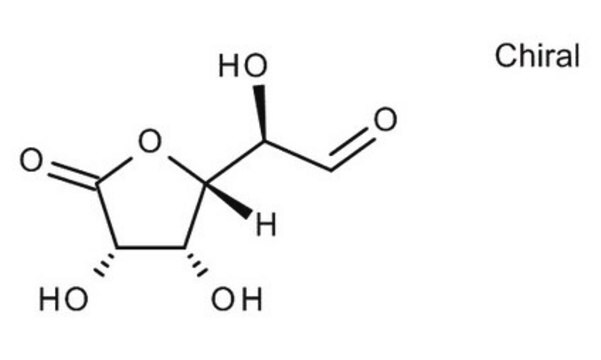
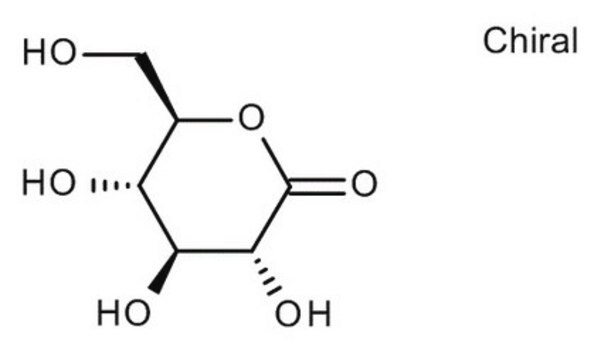
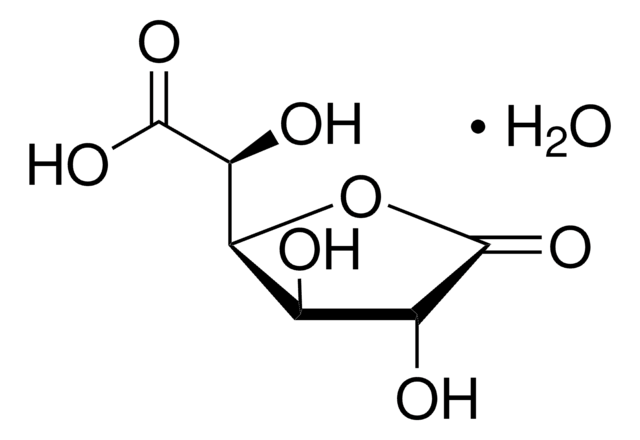
D-(+)-Glucuronic acid γ-lactone
≥99%
Synonym(s):
D-(+)-Glucurono-6,3-lactone, D-Glucurone, D-Glucurono-6,3-lactone, Glucuronolactone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H8O6
CAS Number:
Molecular Weight:
176.12
Beilstein:
83595
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
optical activity
[α]24/D +18.8°, c = 8 in H2O
mp
172-175 °C (lit.)
solubility
water: soluble 25 mg/mL, clear, colorless
SMILES string
O=C([C@@H]([C@@H](O1)[C@H](O)[C@H](O)C1=O)O)[H]
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h1-5,8-10H/t2-,3+,4-,5+/m0/s1
InChI key
UYUXSRADSPPKRZ-SKNVOMKLSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
D-(+)-Glucuronic acid γ-lactone (Glucourono-γ-lactone, Glucurone or Glycurone) is a carbohydrate derivative. It converted into L-ascorbic acid in animals and human body. Its molecule contains two five-membered rings. Its crystal structure has been studied.
Application
D-(+)-Glucuronic acid γ-lactone may be used in the following studies:
- As starting ragent in the synthesis of 2,3,4,-tris(tert.-butyldimethysilyl) glucuronic acid trichloroethylester, required for the preparation of 1-O-acyl glucuronide of the anti-inflammatory drug ML-3000.
- Synthesis of optically active glucopyranoses.
- Synthesis of long-chain alkyl glucofuranosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Alford et al.
Amino acids, 21(2), 139-150 (2001-10-23)
The effects of Red Bull Energy Drink, which includes taurine, glucuronolactone, and caffeine amongst the ingredients, were examined over 3 studies in a total of 36 volunteers. Assessments included psychomotor performance (reaction time, concentration, memory), subjective alertness and physical endurance.
Rec. Trav. Chim., 113, 79-79 (1994)
Benjamin J Ayers et al.
The Journal of organic chemistry, 77(18), 7777-7792 (2012-08-30)
The enantiomers of glucuronolactone are excellent chirons for the synthesis of the 10 stereoisomeric 2,5-dideoxy-2,5-iminohexitols by formation of the pyrrolidine ring by nitrogen substitution at C2 and C5, with either retention or inversion of configuration; the stereochemistry at C3 may
Andreas F G Glawar et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(30), 9341-9359 (2012-06-28)
The efficient scalable syntheses of 2-acetamido-1,2-dideoxy-D-galacto-nojirimycin (DGJNAc) and 2-acetamido-1,2-dideoxy-D-gluco-nojirimycin (DNJNAc) from D-glucuronolactone, as well as of their enantiomers from L-glucuronolactone, are reported. The evaluation of both enantiomers of DNJNAc and DGJNAc, along with their N-alkyl derivatives, as glycosidase inhibitors showed
A one-step C-linked disaccharide synthesis from carbohydrate allylsilanes and tri-O-acetyl-D-glucal.
A de Raadt et al.
Carbohydrate research, 220, 101-115 (1991-11-11)
The reaction of protected glucuronic esters 2 and 7, as well as D-glucuronolactone derivative 11, with (trimethylsilyl)methylmagnesium chloride in ether led to the corresponding stable bis-silyl adducts 3, 8, and 12, respectively. In Peterson-type reactions catalysed with mild acid, these
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 851450-1KG-A | 4061826736517 |
| 851450-100G-A | 4061833023570 |
| 851450-25G-A |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service