807761
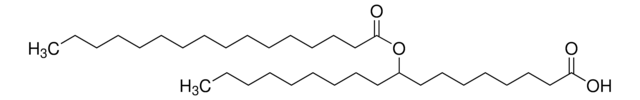
palmitic-acid-5-hydroxy-stearic-acid
95%
Synonym(s):
5-PAHSA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C34H66O4
CAS Number:
Molecular Weight:
538.89
UNSPSC Code:
12352106
Recommended Products
General description
Palmitic-acid-5-hydroxy-stearic-acid (5-PAHSA) is also referred to as 5-hexadecanoyloxyoctadecanoic acid (IUPAC name).
Application
5-PAHSA is composed of palmitic acid attached to stearic acid and is in the class of branched lipids referred to as fatty acid esters of hydroxy fatty acids (FAHFAs). 5-PAHSA was found at reduced levels in adipose tissue and serum of insulin-resistant mice. Levels were also found to be lower in white adipose tissue and serum of humans. Studies of oral administration of 5-PAHSA to obese, diabetic mice showed improved glucose tolerance. In characterizing the mechanism, it was found that 5-PAHSA stimulates insulin and GLP-1 secretion and also enhances glucose transport and the Glut4 transporter through activation of GPR-120.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mark M Yore et al.
Cell, 159(2), 318-332 (2014-10-11)
Increased adipose tissue lipogenesis is associated with enhanced insulin sensitivity. Mice overexpressing the Glut4 glucose transporter in adipocytes have elevated lipogenesis and increased glucose tolerance despite being obese with elevated circulating fatty acids. Lipidomic analysis of adipose tissue revealed the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service