798436
Endo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine
Synonym(s):
(1S,4S,5S)-5-(naphthalen-1-yl)-2-tosyl-2-aza-5-phosphabicyclo[2.2.1]heptane
About This Item
Recommended Products
form
powder
Quality Level
storage condition
under inert gas
storage temp.
2-8°C
SMILES string
O=S(N1C[C@H]2[P@@](C3=CC=CC4=C3C=CC=C4)C[C@@H]1C2)(C5=CC=C(C)C=C5)=O
InChI
1S/C22H22NO2PS/c1-16-9-11-20(12-10-16)27(24,25)23-14-19-13-18(23)15-26(19)22-8-4-6-17-5-2-3-7-21(17)22/h2-12,18-19H,13-15H2,1H3
InChI key
RGHUOMJULXTHPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Other Notes
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(11bS)-4,5-Dihydro-3H-dinaphtho[2,1-c:1′,2′-e]phosphepine 97%](/deepweb/assets/sigmaaldrich/product/structures/375/473/600ccee4-aecf-467e-95fb-922ec56356f3/640/600ccee4-aecf-467e-95fb-922ec56356f3.png)
![Exo-2-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/324/907/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4/640/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4.png)
![Endo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/060/39f2b621-f484-49c3-b692-cdb610e8c517/640/39f2b621-f484-49c3-b692-cdb610e8c517.png)
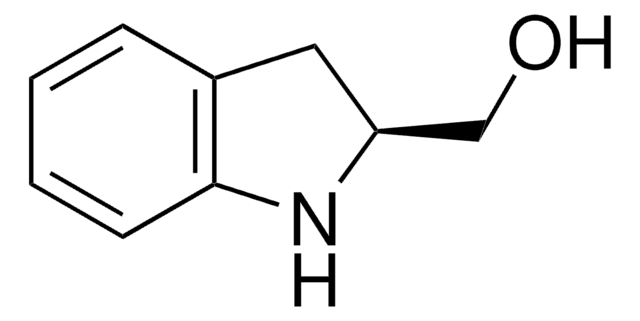
![Exo-4-anisole Kwon [2.2.1] bicyclic phosphine](/deepweb/assets/sigmaaldrich/product/structures/114/753/2a544671-b0e0-4556-8dc3-46c126d6c8ab/640/2a544671-b0e0-4556-8dc3-46c126d6c8ab.png)
![Exo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![Calix[4]arene-25,26,27,28-tetrol 95%](/deepweb/assets/sigmaaldrich/product/structures/198/765/9972559b-b50f-4745-b1fd-9a468e47ef55/640/9972559b-b50f-4745-b1fd-9a468e47ef55.png)
![(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)
![Tris[3-(trimethoxysilyl)propyl] isocyanurate technical grade](/deepweb/assets/sigmaaldrich/product/structures/239/690/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf/640/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf.png)
![Endo-4-Methoxyphenyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/404/012/38bdf2c6-e120-483d-8c3c-8fa3b328963c/640/38bdf2c6-e120-483d-8c3c-8fa3b328963c.png)
