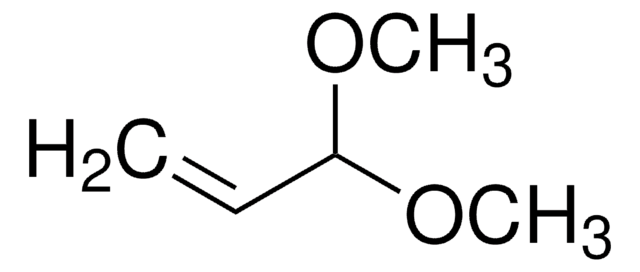
677213
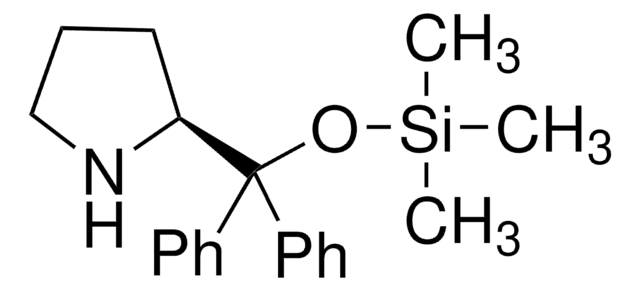
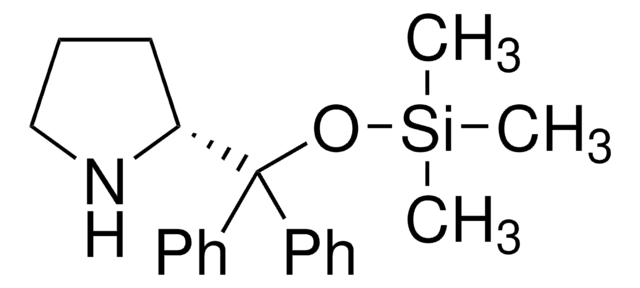
(R)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether
technical grade
Synonym(s):
(R)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]prolinol trimethylsilyl ether, (R)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)]trimethylsilanyloxy)methyl]pyrrolidine, (R)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)]trimethylsilyloxy)methyl]pyrrolidine
About This Item
Recommended Products
grade
technical grade
optical purity
enantiomeric excess: ≥99.0% (HPLC)
mp
46-55 °C
functional group
fluoro
SMILES string
C[Si](C)(C)OC([C@H]1CCCN1)(c2cc(cc(c2)C(F)(F)F)C(F)(F)F)c3cc(cc(c3)C(F)(F)F)C(F)(F)F
InChI
1S/C24H23F12NOSi/c1-39(2,3)38-20(19-5-4-6-37-19,13-7-15(21(25,26)27)11-16(8-13)22(28,29)30)14-9-17(23(31,32)33)12-18(10-14)24(34,35)36/h7-12,19,37H,4-6H2,1-3H3/t19-/m1/s1
InChI key
MOHRGTBNEJKFMB-LJQANCHMSA-N
Related Categories
Application
- Cyclocondensation of enals with methylenepyrrolidines
- Organocatalytic additions of β-ketosulfoxides to conjugated aldehydes
- Organocatalytic aza-Michael reactions
- Stereoselective propargylic alkylation of propargylic esters with aldehydes
- Epoxidation or aziridination of α,β-unsaturated aldehydes and Feist-Benary reactions of 1,3-dicarbonyls
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Geoffrey Coates and co-workers at Cornell University have reported the preparation and use of catalysts composed of an oxophilic Lewis acid and a cobalt tetracarbonyl anion for the ring expansive carbonylation of epoxides to b-lactones and b-lactones to succinic anhydrides.
Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)

![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)
![(S)-2-[[3,5-Bis(trifluoromethyl)phenyl]thioureido]-N-benzyl-N,3,3-trimethylbutanamide 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)