All Photos(1)
About This Item
Empirical Formula (Hill Notation):
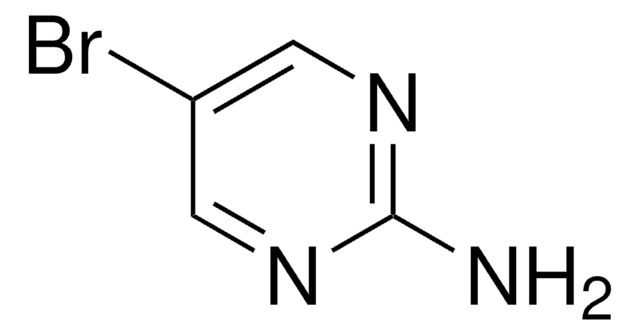
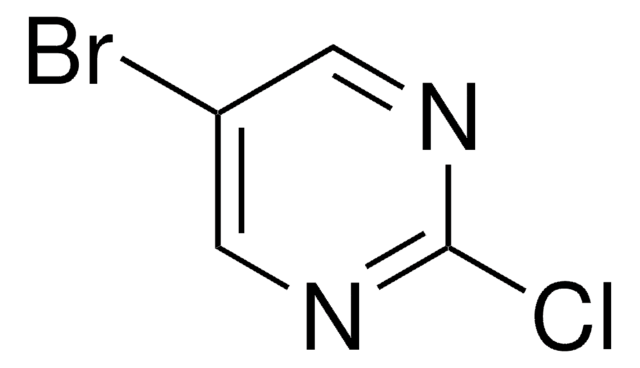
C4H4BrN3
CAS Number:
Molecular Weight:
174.00
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
113-117 °C (lit.)
functional group
bromo
SMILES string
Nc1cnc(Br)cn1
InChI
1S/C4H4BrN3/c5-3-1-8-4(6)2-7-3/h1-2H,(H2,6,8)
InChI key
KRRTXVSBTPCDOS-UHFFFAOYSA-N
Application
Used without prior protection of the amino group in a palladium-catalyzed cross-coupling with pyridylboronic acids leading to pyrazinylpyridines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Amy E Thompson et al.
The Journal of organic chemistry, 70(1), 388-390 (2004-12-31)
A range of halogenated aromatics and heteroaromatics bearing a primary amine group are shown to be suitable substrates for Suzuki cross-coupling reactions with arylboronic acids and pyridylboronic acids under standard conditions, without the need for protection/deprotection steps. New amino-substituted arylpyridines
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service