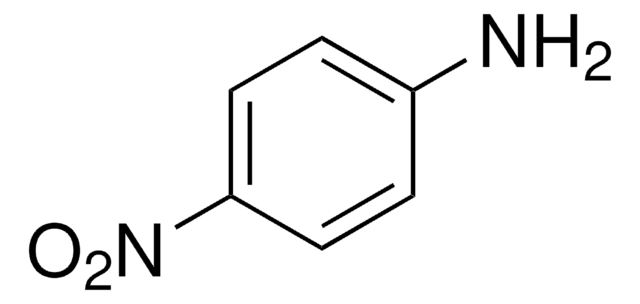
564613
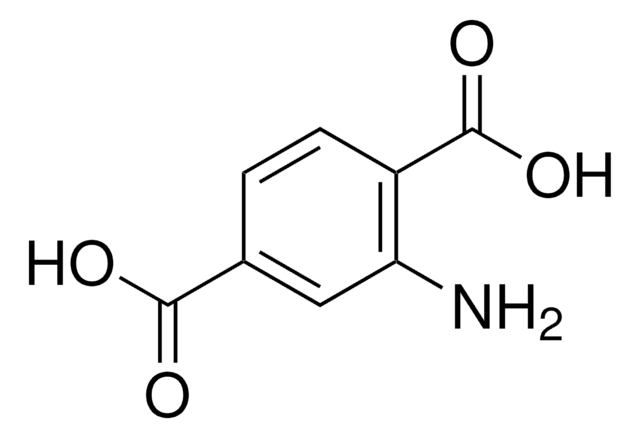
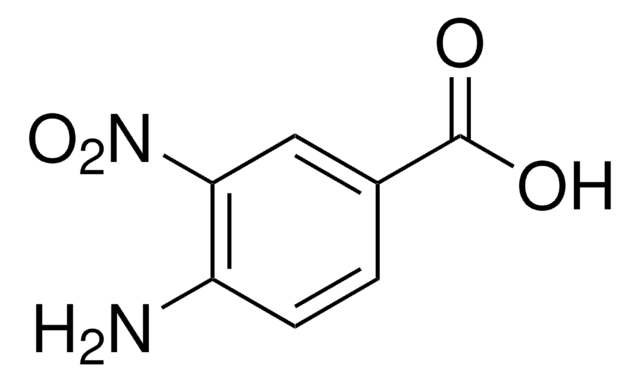
5-Amino-2-nitrobenzoic acid
97%
Synonym(s):
2-Nitro-5-aminobenzoic acid, 3-Carboxy-4-nitroaniline, NSC 74455
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
182.13
Beilstein:
2107512
EC Number:
MDL number:
UNSPSC Code:
12352106
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
236-238 °C (lit.)
application(s)
peptide synthesis
SMILES string
Nc1ccc(c(c1)C(O)=O)[N+]([O-])=O
InChI
1S/C7H6N2O4/c8-4-1-2-6(9(12)13)5(3-4)7(10)11/h1-3H,8H2,(H,10,11)
InChI key
KZZWQCKYLNIOBT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reactant for:
- Two-component dendritic chain reactions
- Enzymic activation of hydrophobic self-immolative dendrimers
- Preparation of insulin receptor tyrosine kinase activator
- Preparation of polymer-bound diazonium salts using Merrifield resin-bound piperazine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Ox. Sol. 2
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Eran Sella et al.
Chemical communications (Cambridge, England), 46(35), 6575-6577 (2010-08-18)
A new dendritic chain reaction probe system was demonstrated to produce exponential signal amplification for the detection of sulfhydryl compounds.
L C Kirby et al.
Lipids, 30(9), 863-867 (1995-09-01)
Conjugated bile acid hydrolase (CBAH) refers to a class of enzymes which catalyze the cleavage of the amino acid moieties from conjugated bile acids. These enzymes are significant because of their role in providing substrates for further microbial metabolism in
Yu Jin Park et al.
Clinical biochemistry, 50(12), 719-725 (2017-03-05)
The use of iodinated contrast media has grown in popularity in the past two decades, but relatively little attention has been paid to the possible interferential effects of contrast media on laboratory test results. Herein, we investigate medical contrast media
Magdalena Wysocka et al.
Analytical biochemistry, 399(2), 196-201 (2010-01-16)
Previously selected by the combinatorial chemistry approach, potent fluorogenic substrate of proteinase 3 was used as the starting structure to design new substrates. The general formula of the synthesized peptides is as follows: ABZ-Tyr-Tyr-Abu-ANB-X-NH(2), where ANB (5-amino-2-nitrobenzoic acid) served as
F Schiele et al.
Clinical chemistry, 33(11), 1978-1982 (1987-11-01)
We describe the process of certification for a gamma-glutamyltransferase reference material (CRM no. 319). Fifteen laboratories participated to this interlaboratory evaluation. All steps of the measurements were controlled in an effort to locate potential sources of variations. In particular, the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service