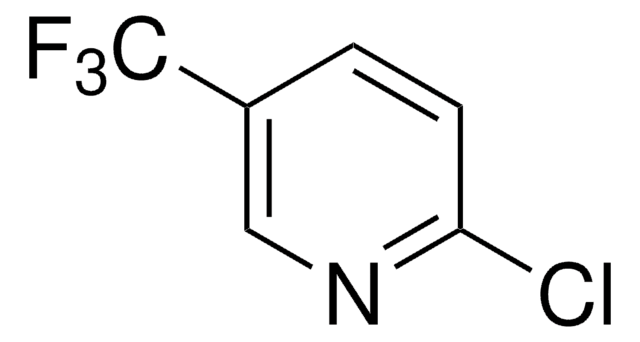
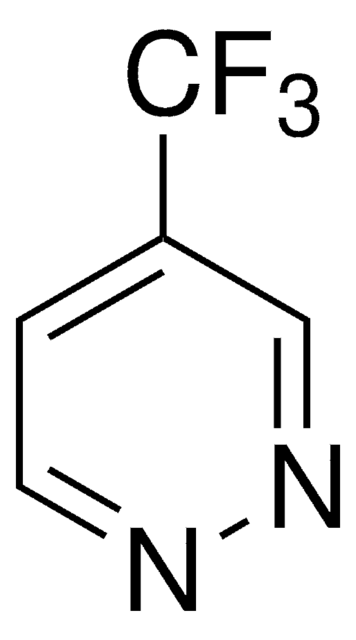
522910
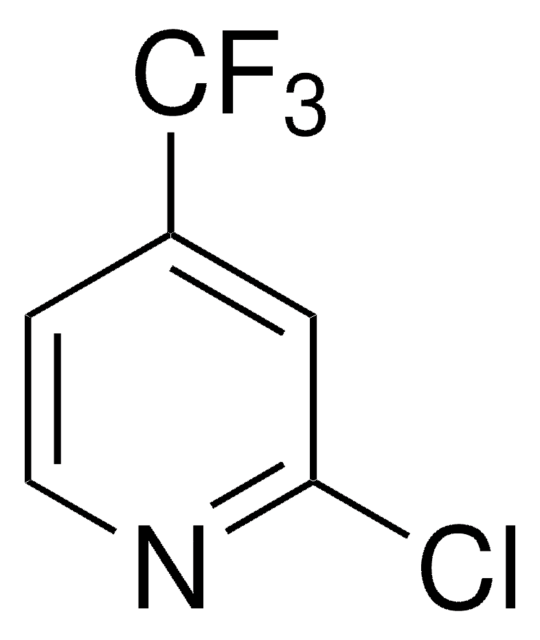
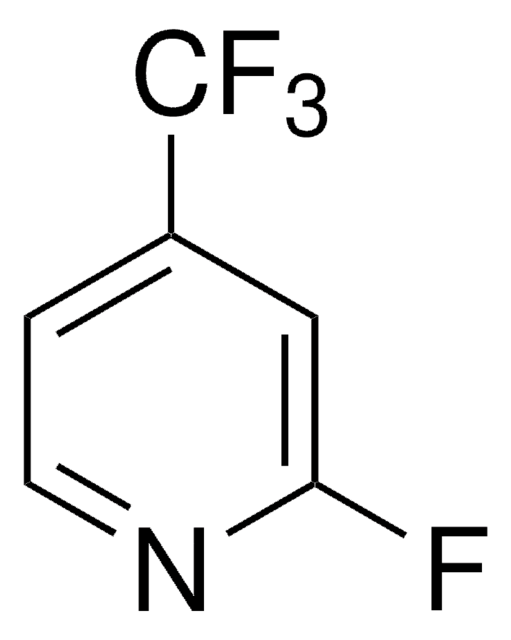
4-(Trifluoromethyl)pyridine
97%
Synonym(s):
4-(Trifluoromethyl)pyridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4F3N
CAS Number:
Molecular Weight:
147.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.417 (lit.)
bp
110 °C (lit.)
density
1.27 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)c1ccncc1
InChI
1S/C6H4F3N/c7-6(8,9)5-1-3-10-4-2-5/h1-4H
InChI key
IIYVNMXPYWIJBL-UHFFFAOYSA-N
General description
4-(Trifluoromethyl)pyridine is a pyridine derivative. It can be prepared by trifluoromethylation of 4-iodobenzene.
Application
4-(Trifluoromethyl)pyridine may be used in the following:
- Preparation of (trifluoromethyl)pyridyllithiums, via metalation reaction.
- Synthesis of metal-organic frameworks (MOFs).
- Synthesis of methiodide salts.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Manfred Schlosser et al.
Chemical Society reviews, 36(7), 1161-1172 (2007-06-20)
Pyridines carrying heterosubstituents (such as carboxy, amido, amino, alkoxy or trifluoromethyl groups or solely individual halogen atoms) can be readily and site selectively metalated. Subsequent reaction with a suitable electrophile opens rational access to a wealth of new building blocks
Fluorinated pyridine derivatives: Part 1. The synthesis of some mono-and bis-quaternary pyridine salts of potential use in the treatment of nerve agent poisoning.
Timperley CM, et al.
Journal of Fluorine Chemistry, 126(8), 1160-1165 (2005)
Enhancement of CO2/N2 selectivity in a metal-organic framework by cavity modification.
Bae YS, et al.
Journal of Materials Chemistry, 19(15), 2131-2134 (2009)
Copper-mediated trifluoromethylation of heteroaromatic compounds by trifluoromethyl sulfonium salts.
Cheng-Pan Zhang et al.
Angewandte Chemie (International ed. in English), 50(8), 1896-1900 (2011-02-18)
Malcolm E Tessensohn et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(16), 2250-2257 (2017-06-14)
The voltammetric behavior of 2,3,5,6-tetramethyl-1,4-phenylenediamine was found to be able to differentiate the hydrogen acceptor abilities of electroinactive pyridine compounds in acetonitrile. Weak and strong hydrogen acceptors were distinguished through the onset of a third oxidation process that came about
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service