470198
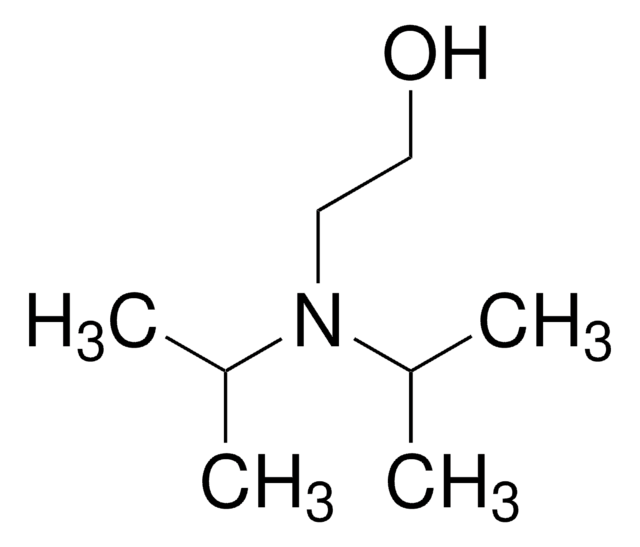
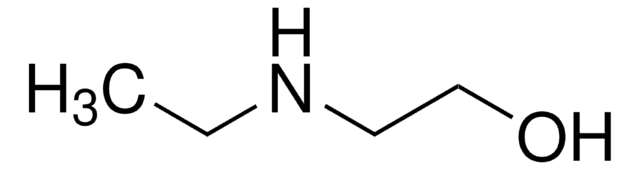
2-(Isopropylamino)ethanol
70%
Synonym(s):
N-Isopropylethanolamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHNHCH2CH2OH
CAS Number:
Molecular Weight:
103.16
Beilstein:
1633453
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
70%
impurities
30% N-isopropyl-2,2′-iminodiethanol
refractive index
n20/D 1.441 (lit.)
bp
172 °C (lit.)
density
0.897 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
CC(C)NCCO
InChI
1S/C5H13NO/c1-5(2)6-3-4-7/h5-7H,3-4H2,1-2H3
InChI key
RILLZYSZSDGYGV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-(Isopropylamino)ethanol is a secondary amine. The solubility of CO2 in aqueous solutions of 2-(isopropylamino)ethanol (IPAE) has been evaluated.
Application
2-(Isopropylamino)ethanol may be used in the synthesis of heterocyclic N,O-acetals.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
172.4 °F - closed cup
Flash Point(C)
78 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Menegola et al.
Reproductive toxicology (Elmsford, N.Y.), 12(3), 371-374 (1998-06-17)
Previously, postcoital antifertility effects of a number of aminoalcohols, including 2-(isopropylamino)-ethanol, have been demonstrated in rodents. In this experiment, we compared the antifertility activity of 2-(isopropylamino)-ethanol to the following analogs: hydroxyethylpiperidine, hydroxyethylpiridine, hydroxyethylpirrolidine, and hydroxyethylpirrolidone. Female rats were gavaged on
Saba S, et al.
Journal of Chemical Education, 84, 1011-1011 (2007)
L S Borman
In vitro, 18(2), 129-140 (1982-02-01)
The choline analog, N-isopropylethanolamine (IPE), inhibits the growth of both Chinese hamster ovary CHO-K1 and mouse L-M cells by two kinetically distinct mechanisms; I, a reversible and concentration-dependent reduction in the logarithmic population doubling rate and the saturation density of
CO2 solubility and species distribution in aqueous solutions of 2-(isopropylamino) ethanol and its structural isomers.
Yamada H, et al.
International Journal of Greenhouse Gas Control, 17, 99-105 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service