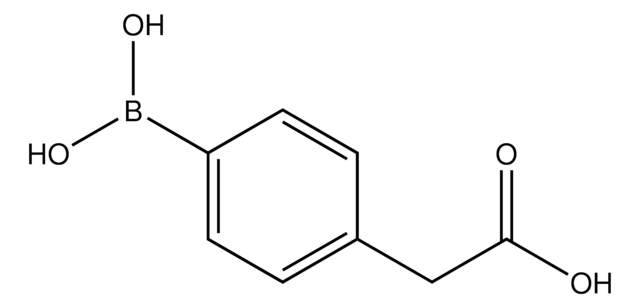
456764
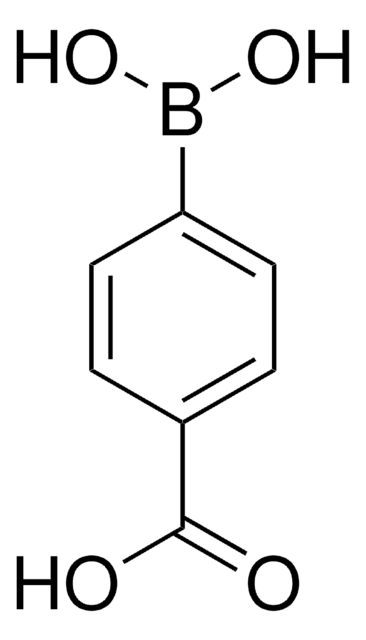
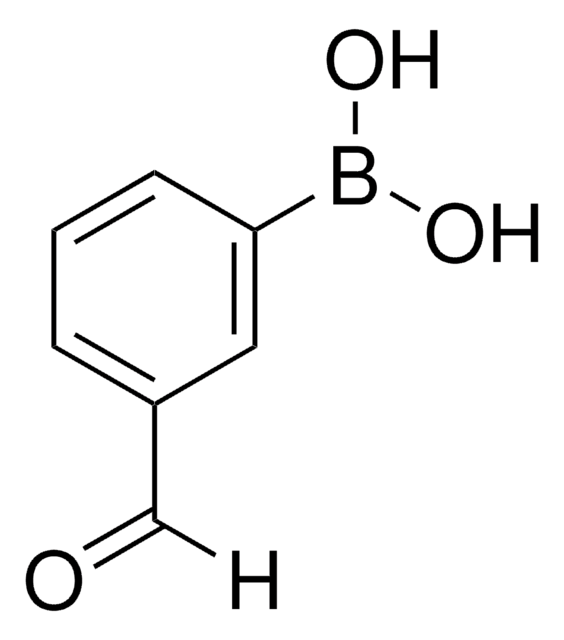
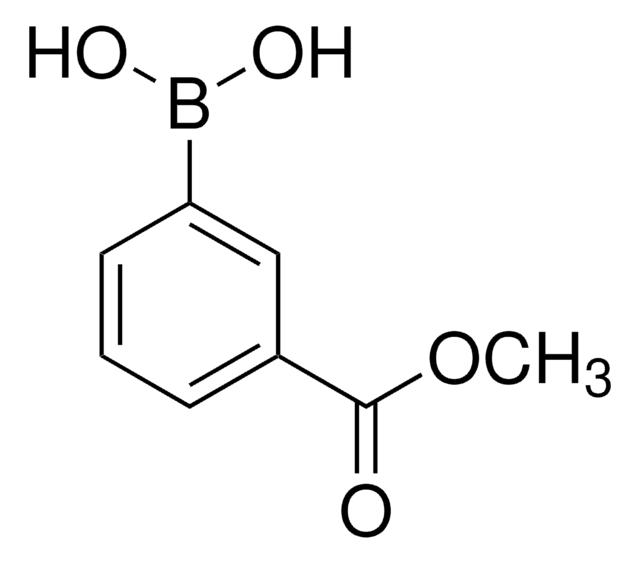
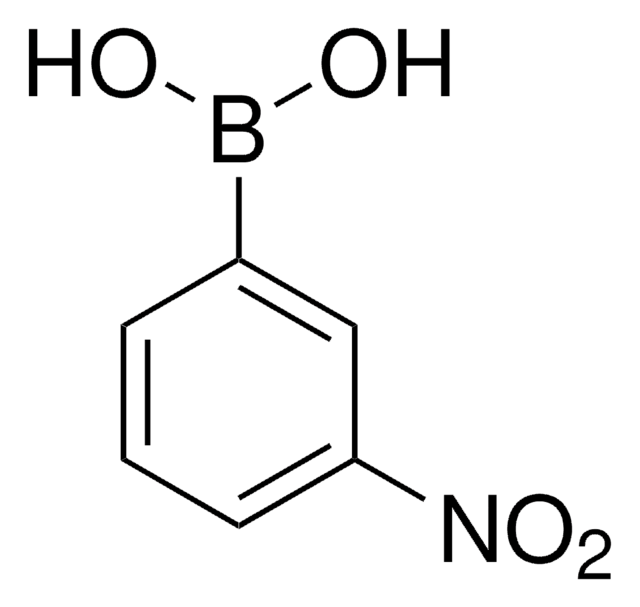
3-Carboxyphenylboronic acid
≥95%
Synonym(s):
μ-Carboxyphenylboronic acid, 3-(Dihydroxyborane)benzoic acid, 3-(Dihydroxyboryl)benzoic acid, 3-Boronobenzoic acid, 3-Carboxybenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HO2CC6H4B(OH)2
CAS Number:
Molecular Weight:
165.94
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
mp
243-247 °C (lit.)
SMILES string
OB(O)c1cccc(c1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,11-12H,(H,9,10)
InChI key
DBVFWZMQJQMJCB-UHFFFAOYSA-N
Application
3-Carboxyphenylboronic acid can be used as a substrate in the preparation of:
- Biaryl derivatives by reacting with bromoaniline through the Suzuki-Miyaura coupling reaction.
- Boronic acid-functionalized block copolymer.
- 1H-Imidazo[1,2-a]quinoxaline derivatives.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of a phenylboronic acid-functionalized thermosensitive block copolymer and its application in separation and purification of vicinal-diol-containing compounds
Wang Y, et al.
Royal Society of Chemistry Advances, 6(85), 82309-82320 (2016)
Di Wu et al.
Acta biomaterialia, 96, 123-136 (2019-06-28)
Locoregional chemotherapy, especially using implantable hydrogel depots to sustainably deliver chemotherapeutics at tumor site, has shown great potential for improving antitumor efficacy and reducing systemic toxicity. However, the hydrogel applications are limited by some intrinsic constraints, especially the contradiction between
Jumin Yang et al.
Materials science & engineering. C, Materials for biological applications, 116, 111250-111250 (2020-08-19)
Various nanoparticles as drug delivery system provide significant improvements in the cancer treatment. However, their clinical success remains elusive in large part due to their inability to overcome both systemic and tumor tissue barriers. The nanosystems with nanoproperty-transformability (surface, size
Novel rhodamine dyes via Suzuki coupling of xanthone triflates with arylboroxins
Calitree, B. D.; Detty, M. R.
Synlett, 89-92 (2010)
New imidazo [1, 2-a] quinoxaline derivatives: synthesis and in vitro activity against human melanoma
Deleuze-Masquefa C, et al.
European Journal of Medicinal Chemistry, 44(9), 3406-3411 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service