450359
N-Boc-aniline
97%
Synonym(s):
tert-Butyl-N-phenylcarbamate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
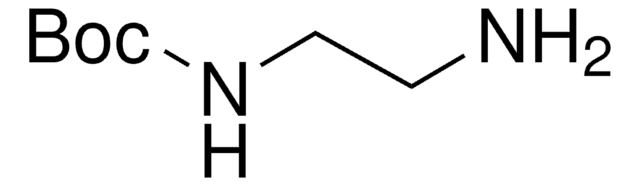
C6H5NHCO2C(CH3)3
CAS Number:
Molecular Weight:
193.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
133-137 °C (lit.)
functional group
amine
SMILES string
CC(C)(C)OC(=O)Nc1ccccc1
InChI
1S/C11H15NO2/c1-11(2,3)14-10(13)12-9-7-5-4-6-8-9/h4-8H,1-3H3,(H,12,13)
InChI key
KZZHPWMVEVZEFG-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
SNAr reactions of benzaldimines: a concise synthesis of substituted phenanthridines.
Reuter DC, et al.
Tetrahedron Letters, 35(28), 4899-4902 (1994)
Directed ortho lithiation of phenylcarbamic acid 1, 1-dimethylethyl ester (N-BOC-aniline). Revision and improvements.
Stanetty P, et al.
The Journal of Organic Chemistry, 57(25), 6833-6837 (1992)
Catalyst-free water-mediated N-Boc deprotection.
Wang G, et al.
Tetrahedron Letters, 50(13), 1438-1440 (2009)
Novel practical deprotection of N-Boc compounds using fluorinated alcohols.
Choy J, et al.
Synthetic Communications, 38(21), 3840-3853 (2008)
Total synthesis of the polycyclic fungal metabolite (+/-)-communesin F.
Peng Liu et al.
Angewandte Chemie (International ed. in English), 49(11), 2000-2003 (2010-02-23)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![4-[(N-Boc)aminomethyl]aniline 97%](/deepweb/assets/sigmaaldrich/product/structures/341/155/530c425c-7e6e-435e-a28a-9d40b05b938a/640/530c425c-7e6e-435e-a28a-9d40b05b938a.png)