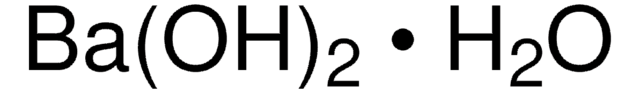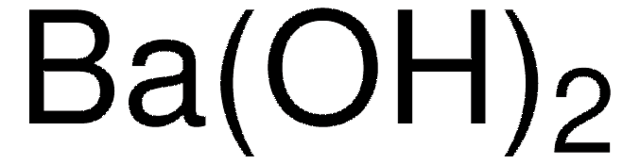433373
Barium hydroxide
technical grade, ~95%
Synonym(s):
Barium dihydroxide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
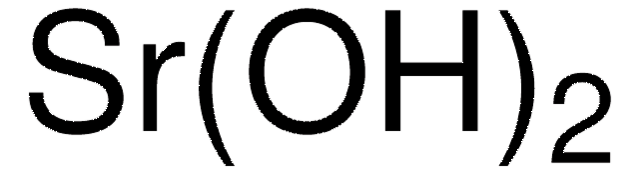
Ba(OH)2
CAS Number:
Molecular Weight:
171.34
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
technical grade
Quality Level
Assay
~95%
form
powder
mp
>300 °C (lit.)
density
2.2 g/mL at 25 °C (lit.)
SMILES string
O[Ba]O
InChI
1S/Ba.2H2O/h;2*1H2/q+2;;/p-2
InChI key
RQPZNWPYLFFXCP-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Reagent used in wet chemical gel-to-crystallite conversion to form BaTiO3 at low temperatures.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kutty, T.R.N. Padmini, P.
Materials Chemistry and Physics, 39, 200-200 (1995)
M F Byford
The Biochemical journal, 280 ( Pt 1), 261-265 (1991-11-15)
The beta-elimination of phosphoserine residues by dilute alkali is catalysed by the presence of group II metal ions. The use of 0.1 M-Ba (OH)2 catalysed the rate of beta-elimination of phosphoserine by more than two orders of magnitude compared with
Spontaneous ignition, explosion, and fire with sevoflurane and barium hydroxide lime.
Junzheng Wu et al.
Anesthesiology, 101(2), 534-537 (2004-07-28)
Marshall B Dunning et al.
Anesthesiology, 106(1), 144-148 (2007-01-02)
Fires, explosions, and extreme heat production may occur when sevoflurane reacts with desiccated barium hydroxide lime. The identity of the flammable gas has not previously been published, although carbon monoxide, methanol, formaldehyde, and methyl formate have been identified in low
P J Baxter et al.
Anesthesiology, 89(4), 929-941 (1998-10-20)
Desflurane, enflurane and isoflurane can be degraded to carbon monoxide (CO) by carbon dioxide absorbents, whereas sevoflurane and halothane form negligible amounts of CO. Carbon monoxide formation is greater with drier absorbent, and with barium hydroxide, than with soda lime.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service