All Photos(1)
About This Item
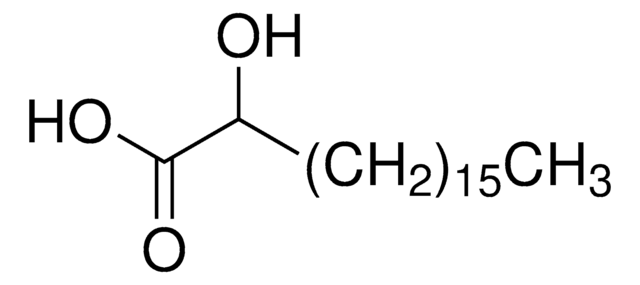
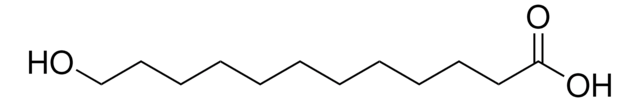
Linear Formula:
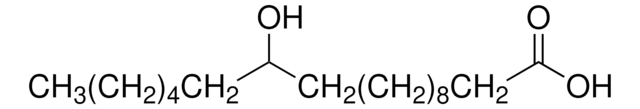
HO(CH2)14CO2H
CAS Number:
Molecular Weight:
258.40
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
85-89 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OCCCCCCCCCCCCCCC(O)=O
InChI
1S/C15H30O3/c16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15(17)18/h16H,1-14H2,(H,17,18)
InChI key
BZUNJUAMQZRJIP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
15-Hydroxypentadecanoic acid is an ω-hydroxy acid. One of the method reported for its synthesis is from 1,12-dodecanolide. It is reported to be one of the bioactive component in Tagetes erecta L. leaf and flower extract.
15-Hydroxypentadecanoic acid undergoes lactonization reaction catalyzed by Mucor javanicus L46 and Mucor miehei to afford macrocyclic mono- and oligolactone derivatives. Its lipase-catalyzed synthesis from 15-tetracosenoic acid in Malania Olcifera Chum oil has been proposed. It also participates in the biosynthesis of pentadecanolide.
Application
15-Hydroxypentadecanoic acid is suitable reagent used in the following studies:
- As an internal standard in the quantification of formation of 11-hydroxylauric acid by gas chromatography.
- In the synthesis of [16-14C]16DCA (DCA= dicarboxylic acid) by one-carbon elongation procedure at C15.
- As an internal standard for the normalization of intensities in the mass spectra of plant cutin polymer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Screening and evaluation of bioactive components of Tagetes erecta L. by GC-MS analysis.
Devika R and Kovilpillai J.
Asian Journal of Pharmaceutical and Clinical Research, 7(2), 58-60 (2014)
Zeolite-catalyzed macrolactonization of Ookoshi T and Onaka M. ω-hydroxyalkanoic acids in a highly concentrated solution.
Ookoshi T and Onaka M.
Tetrahedron Letters, 39(3), 293-296 (1998)
Dušan Veličković et al.
The Plant journal : for cell and molecular biology, 80(5), 926-935 (2014-10-04)
The cutin polymers of different fruit cuticles (tomato, apple, nectarine) were examined using matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI) after in situ release of the lipid monomers by alkaline hydrolysis. The mass spectra were acquired from each coordinate
Preparation of 15-hydroxypentadecanoic acid by means of condensation reaction via β-ketosulfoxide.
Nozaki H, et al.
Canadian Journal of Chemistry, 46(23), 3767-3770 (1968)
Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones.
Antczak U, et al.
Enzyme and Microbial Technology, 13(7), 589-593 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![2-[2-(2-Methoxyethoxy)ethoxy]acetic acid technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)