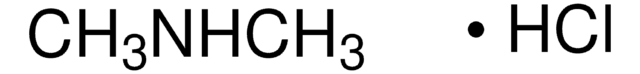391956
Dimethylamine solution
2.0 M in THF
Synonym(s):
N,N-Dimethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2NH
CAS Number:
Molecular Weight:
45.08
Beilstein:
605257
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
concentration
1.90-2.20 M (by NaOH, titration)
2.0 M in THF
bp
~60 °C
density
0.85 g/mL at 25 °C
SMILES string
CNC
InChI
1S/C2H7N/c1-3-2/h3H,1-2H3
InChI key
ROSDSFDQCJNGOL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dimethylamine solution (2M in THF) has been used in the synthesis of 2-dimethylamino-1-(3,4-methylenedioxyphenyl)propan-1-one (bk-MDDMA) , a β-keto derivative of 3,4-methylenedioxyamphetamine (MDA) by reacting with 2-bromo-3′,4′-methylenedioxypropiophenone. It may also be used to synthesize organic intermediates such as dimethyl-(4-nitro-benzyl)-amine, dimethyl-(4-methyl-benzyl)-amine and 4-dimethylamino-but-2-enoic acid [4-(3,4-dichloro-6-fluoro-phenylamino)-quinazolin-6-yl]-amide.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-32.8 °F - closed cup
Flash Point(C)
-36 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[11C]-dimethylamine as a labeling agent for PET biomarkers.
Jacobson O & Mishani E.
Applied Radiation and Isotopes, 66(2), 188-193 (2008)
Discrimination and identification of regioisomeric ?-keto analogues of 3, 4-methylenedioxyamphetamines by gas chromatography-mass spectrometry.
Zaitsu K, et al.
Forensic Toxicology, 26(2), 45-51 (2008)
L Lee et al.
Cancer research, 41(10), 3992-3994 (1981-10-01)
Using a method for nitrosamine analysis that gives high recovery values and that is free from artifactual synthesis of nitrosamines, we have shown that human feces do not contain volatile nitrosamines (detection limit, 0.1 to 0.5 microgram/kg). We also showed
Gerhild Zauner et al.
Biochimica et biophysica acta, 1820(9), 1420-1428 (2011-08-02)
Analysis of protein glycosylation is an important first step towards establishing the functions of glycans in health and disease. In contrast to N-glycans which are generally enzymatically released for analysis, there is no corresponding enzyme for O-glycan liberation. Therefore, O-glycans
George Nicholson et al.
PLoS genetics, 7(9), e1002270-e1002270 (2011-09-21)
We have performed a metabolite quantitative trait locus (mQTL) study of the (1)H nuclear magnetic resonance spectroscopy ((1)H NMR) metabolome in humans, building on recent targeted knowledge of genetic drivers of metabolic regulation. Urine and plasma samples were collected from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service