378151
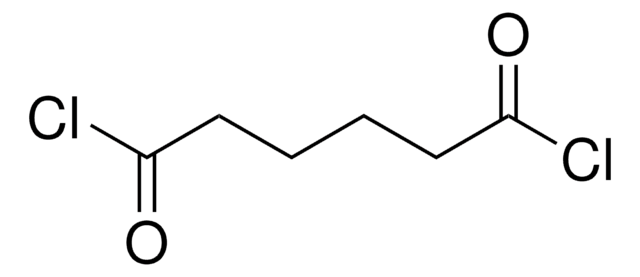
Diglycolyl chloride
95%
Synonym(s):
2,2′-Oxydiacetyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O(CH2COCl)2
CAS Number:
Molecular Weight:
170.98
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.473 (lit.)
bp
84-87 °C/2 mmHg (lit.)
density
1.439 g/mL at 25 °C (lit.)
functional group
acyl chloride
ether
SMILES string
ClC(=O)COCC(Cl)=O
InChI
1S/C4H4Cl2O3/c5-3(7)1-9-2-4(6)8/h1-2H2
InChI key
GTZXSBQCNBNWPK-UHFFFAOYSA-N
General description
Diglycolyl chloride (2,2′-Oxydiacetyl chloride) is an acid halide.
Application
Diglycolyl chloride is suitable for use in the synthesis of ply(ether ester). It may be used in the synthesis of:
- chiral diphenyl substituted polyether-diester compounds
- morpholine dione analog (IMDNQ)
- salicylic acid (SA)- based diacids
Diglycolyl chloride may be used in the synthesis of the following compounds:
- diazadibenzo-18-crown-6 diamide
- diazadi(tert-butylbenzo)-18-crown-6 diamide
- surfen derivative
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of chiral diphenyl substituted polyether-diester compounds.
Bradshaw JS, et al.
The Journal of Organic Chemistry, 47(7), 1229-1232 (1982)
Ashley L Carbone et al.
Macromolecular rapid communications, 30(12), 1021-1021 (2010-02-18)
Fast-degrading, salicylate-based poly(anhydride-esters) were designed to degrade and release the active component, salicylic acid (SA), within 1 week. The polymer degradation was enhanced by using shorter or oxygen-containing aliphatic chains. A copolymer of diglycolic acid was also made with a
Synthesis and CO2 Solubility Studies of Poly (ether carbonate) s and Poly (ether ester) s Produced by Step Growth Polymerization.
Tan B, et al.
Macromolecules, 38(5), 1691-1698 (2005)
J C Aguilar et al.
Talanta, 54(6), 1195-1204 (2008-10-31)
The ligands 4,7-diaza-2,3,8,9-dibenzo-15-crown-5 (L1), 4,10-diaza-2,3,11,12-dibenzo-18-crown-6 (L2), 4,10-diaza-2,3,11,12-di(4'-tert-butylbenzo)-18-crown-6 (L3) and N,N-di(methylenecarboxyethoxy) 4,10-diaza-2,3,11,12-dibenzo-18-crown-6 (L4) have been prepared. Partition coefficients and acid dissociation constants for these four diazadibenzocrown ether compounds were determined in water-chloroform. Their effectiveness was assessed in solvent extraction of Pb(2+)
Small molecule antagonists of cell-surface heparan sulfate and heparin-protein interactions.
Weiss RJ, et al.
Chemical Science, 6(10), 5984-5993 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service