361399
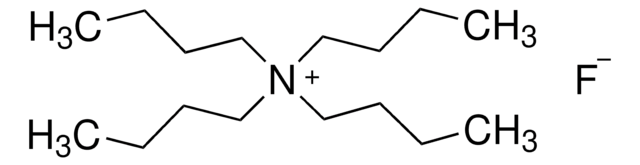
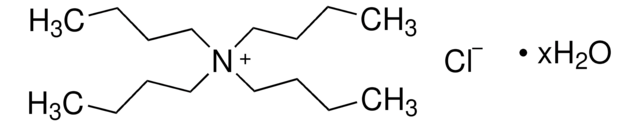
Tetrabutylammonium fluoride solution
75 wt. % in H2O
Synonym(s):
TBAF solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
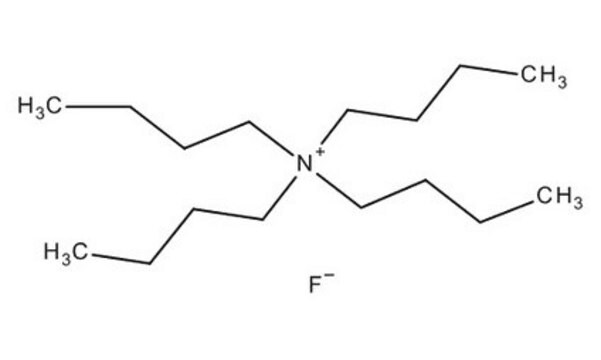
[CH3(CH2)3]4NF
CAS Number:
Molecular Weight:
261.46
Beilstein:
3762762
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
viscous liquid
Quality Level
concentration
75 wt. % in H2O
density
0.953 g/mL at 25 °C
functional group
amine
SMILES string
[F-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.FH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
InChI key
FPGGTKZVZWFYPV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reactant for preparation of:
- Triple monoamine reuptake inhibitors as a new generation of antidepressants
- Alcohols via hydrolysis of alkyl silyl ethers neutral pH in mixed organic-aqueous buffered solutions
- Oligoribonucleotides with phosphonate-modified linkages
- Aryl alkyl alcohols via Nozaki-Hiyama allylation catalyzed by chiral bipyridyldiol ligands and chromium trichloride
- Conjugated dienoic acid esters using Suzuki coupling reactions
- Macrocyclic o-aminobenzamide Hsp90 inhibitorwith antitumor activity
- Phospshoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors
- Anti-diabetic polyacetylenic glucosides
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Supplementary Hazards
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chao Zhong et al.
Carbohydrate polymers, 94(1), 38-45 (2013-04-03)
In this article, a novel and high efficient solvent, tetra-n-Butylammonium Hydroxide (TBAH), was used for dissolution and isolation of straw cellulose from wheat straw. The composition analysis with gas chromatography (GC) and the spectroscopic characterization analysis conducted by X-Ray diffraction
R Bryan Sears et al.
Journal of inorganic biochemistry, 121, 77-87 (2013-01-29)
The complex cis-[Ru(phpy)(phen)(CH3CN)2](+) (phpy=2-phenylpyridine, phen=1,10-phenanthroline) was investigated as a potential photodynamic therapy (PDT) agent. This complex presents desirable photochemical characteristics including a low energy absorption tail extending into the PDT window (600-850nm) and photoinduced exchange of the CH3CN ligands, generating
Oliver Bixner et al.
The Journal of chemical physics, 136(20), 204503-204503 (2012-06-07)
The interaction of exciton and charge transfer (CT) states plays a central role in photo-induced CT processes in chemistry, biology, and physics. In this work, we use a combination of two-dimensional electronic spectroscopy (2D-ES), pump-probe measurements, and quantum chemistry to
Mei Shen et al.
Journal of the American Chemical Society, 134(24), 9856-9859 (2012-06-05)
Here we report on the unprecedentedly high resolution imaging of ion transport through single nanopores by scanning electrochemical microscopy (SECM). The quantitative SECM image of single nanopores allows for the determination of their structural properties, including their density, shape, and
Fabio Galeotti et al.
Journal of chromatography. A, 1284, 141-147 (2013-03-05)
In this study, by using tetrabutylammonium bisulfate as ion-pairing reagent, we were able to separate all the main heparin/heparan sulfate disaccharides generated by the action of heparinases along with the main Hep tetrasaccharide possessing a 3-O-sulfate group on the sulfoglucosamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service