All Photos(1)
About This Item
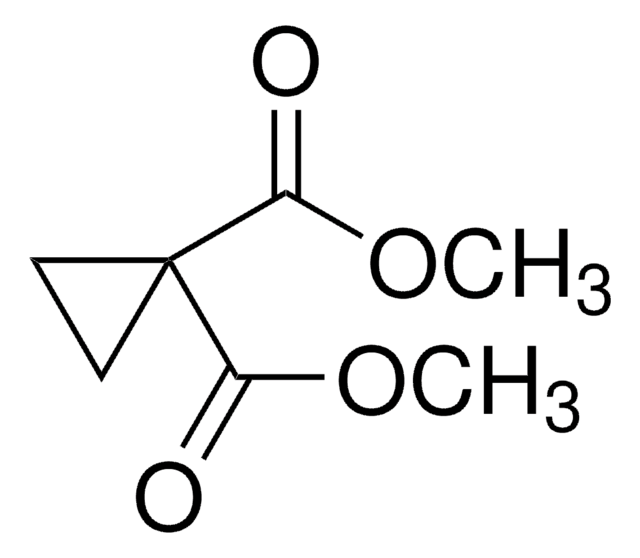
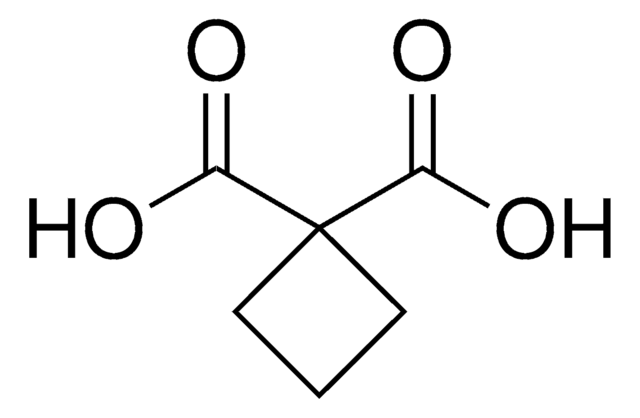
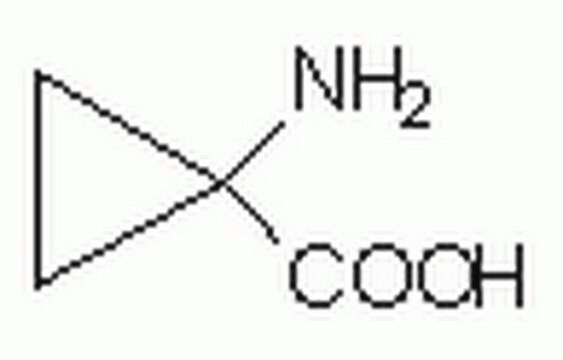
Linear Formula:
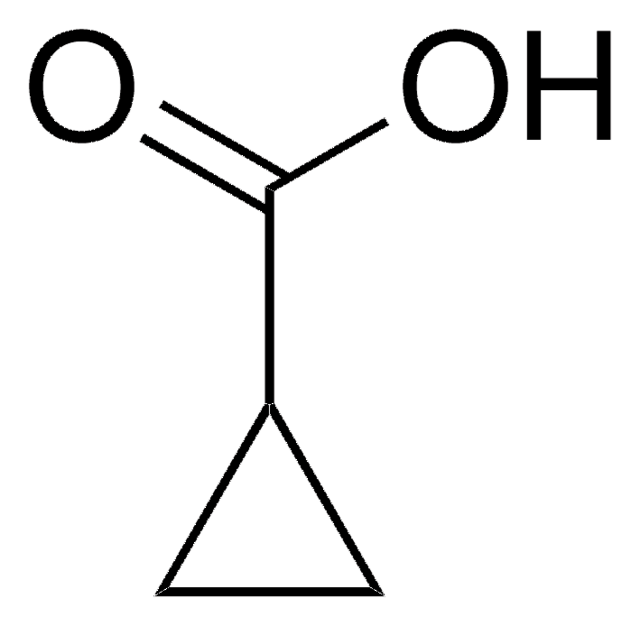
C3H4(CO2H)2
CAS Number:
Molecular Weight:
130.10
Beilstein:
1864823
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
134-136 °C (lit.)
solubility
methanol: soluble 1 g/10 mL, clear, colorless
functional group
carboxylic acid
SMILES string
OC(=O)C1(CC1)C(O)=O
InChI
1S/C5H6O4/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H,6,7)(H,8,9)
InChI key
FDKLLWKMYAMLIF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Cyclopropane-1,1-dicarboxylic acid is a dicarboxylic acid. Cyclopropane-1,1-dicarboxylic acid, an inhibitor of 1-aminocyclopropane-1-carboxylic acid oxidase, was quantitated in Lycopersicum esculentum by HPLC-electrospray tandem mass spectrometry. Crystal and molecular structure of cyclopropane-1,1-dicarboxylic acid has been reported.
Application
Cyclopropane-1,1-dicarboxylic acid was used in the preparation of new heterocyclic derivatives of cyclopropane dicarboxylic acid containing thiadiazole and 1,2,4-triazole moieties. It was also used to prepare spiro-cyclopropyl metallocycles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The crystal and molecular structure of cyclopropane-1, 1-dicarboxylic acid.
Meester MAM, et al.
Acta Crystallographica Section B, Structural Science, 27(3), 630-634 (1971)
Tetrahedron, 45, 1219-1219 (1989)
Paolo Ingallinella et al.
Biochemistry, 41(17), 5483-5492 (2002-04-24)
Serine proteases are the most studied class of proteolytic enzymes and a primary target for drug discovery. Despite the large number of inhibitors developed so far, very few make contact with the prime site of the enzyme, which constitutes an
Nora A Foroud et al.
Phytopathology, 109(5), 796-803 (2018-12-13)
Plant signaling hormones such as ethylene have been shown to affect the host response to various pathogens. Often, the resistance responses to necrotrophic fungi are mediated through synergistic interactions of ethylene (ET) with the jasmonate signaling pathway. On the other
Yan-Biao Kang et al.
Organic & biomolecular chemistry, 4(2), 299-301 (2006-01-05)
A tandem ring-opening-cyclization reaction of cyclopropanes with imines in the presence of 5 mol% of scandium triflate was developed for the highly diastereoselective synthesis of multi-substituted pyrrolidines.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service