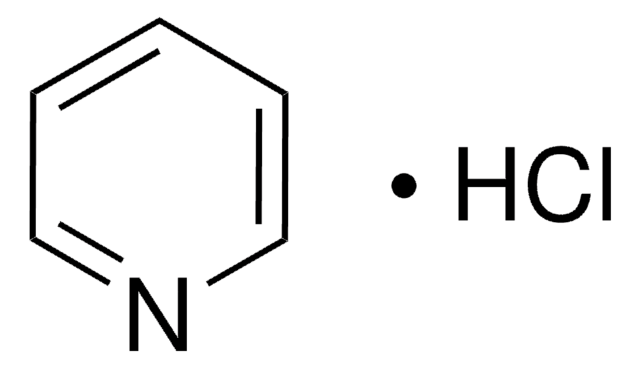
307475
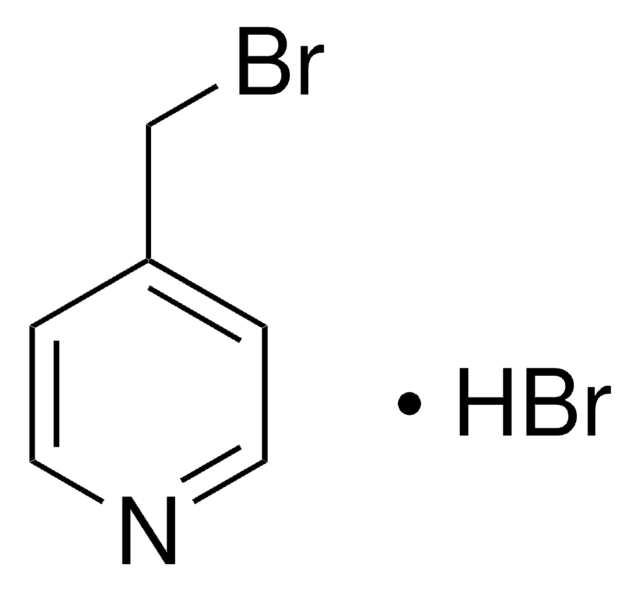
Pyridine hydrobromide
98%
Synonym(s):
Pyridinium bromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H5N · HBr
CAS Number:
Molecular Weight:
160.01
Beilstein:
3615336
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
200 °C (dec.) (lit.)
SMILES string
Br[H].c1ccncc1
InChI
1S/C5H5N.BrH/c1-2-4-6-5-3-1;/h1-5H;1H
InChI key
BBFCIBZLAVOLCF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Pyridine hydrobromide may be used in chemical synthesis studies.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sabyasachi Ta et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 173, 196-200 (2016-09-24)
Combination of pyridine, antipyrine and indole in a single molecule (L2) allows selective recognition of Fe3+ colorimetrically in CH3CN. The structure of L2 is confirmed from single crystal X-ray diffraction analysis. The probe displays two different visible bands at 541nm
Jipan Yu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(13), 4271-4277 (2013-02-13)
Efficient copper-catalyzed aerobic oxidative C-H and C-C functionalization of 1-[2-(arylamino)aryl]ethanones leading to acridones has been developed. The procedure involves cleavage of aromatic C-H and acetyl C-C bonds with intramolecular formation of a diarylketone bond. The protocol uses inexpensive Cu(O2CCF3)2 as
Ye Wei et al.
Journal of the American Chemical Society, 135(10), 3756-3759 (2013-02-27)
We describe here a [3+3]-type condensation reaction of O-acetyl ketoximes and α,β-unsaturated aldehydes that is synergistically catalyzed by a copper(I) salt and a secondary ammonium salt (or amine). This redox-neutral reaction allows modular synthesis of a variety of substituted pyridines
Prasanta Das et al.
The Journal of chemical physics, 138(5), 054307-054307 (2013-02-15)
With infrared absorption spectra we investigated the reaction between Cl atom and pyridine (C(5)H(5)N) in a para-hydrogen (p-H(2)) matrix. Pyridine and Cl(2) were co-deposited with p-H(2) at 3.2 K; a planar C(5)H(5)N-Cl(2) complex was identified from the observed infrared spectrum
Harry Adams et al.
Journal of the American Chemical Society, 135(5), 1853-1863 (2013-01-31)
The association constants for a family of 96 closely related zinc porphyrin-pyridine ligand complexes have been measured in two different solvents, toluene and 1,1,2,2-tetrachloroethane (TCE). The zinc porphyrin receptors are equipped with phenol side arms, which can form intramolecular H-bonds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)