All Photos(4)
About This Item
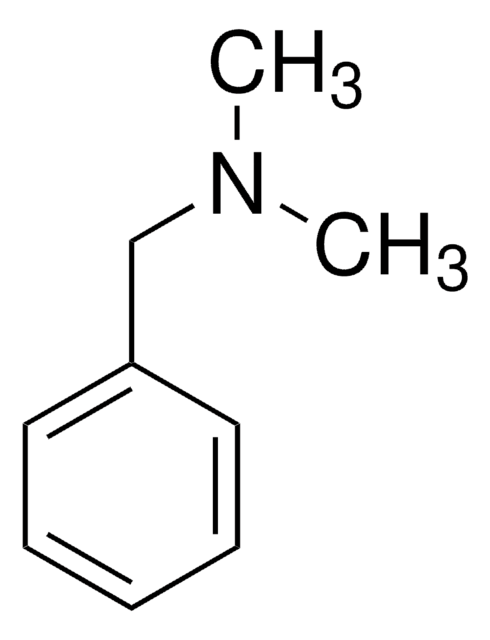
Linear Formula:
C6H5CON(CH3)2
CAS Number:
Molecular Weight:
149.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
132-133 °C/15 mmHg (lit.)
mp
43-45 °C (lit.)
functional group
amide
phenyl
SMILES string
CN(C)C(=O)c1ccccc1
InChI
1S/C9H11NO/c1-10(2)9(11)8-6-4-3-5-7-8/h3-7H,1-2H3
InChI key
IMNDHOCGZLYMRO-UHFFFAOYSA-N
General description
N,N-Dimethylbenzamide is a hydrotropic agent and its ability to solubilize drugs with low aqueous solubility has been investigated. It reacts with PhLnI complexes (Ln-Eu, Sm, Yb) to yield benzophenone in good yields. Deuterium exchange labelling experimets on N,N-dimethylbenzamide using [IrH2(Me2CO)2(PPh3)2]BF4 as catalyst and deuterium gas as the source of isotope has been conducted.
Application
Reagent for deoxygenation of secondary alcohols.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of ethyl-and phenyllanthanide s complexes with n, n-dimethylbenzamide and benzaldehyde.
Yokoo K, et al.
Polyhedron, 2(10), 1101-1102 (1983)
Deuterium exchange labelling of substituted aromatics using [IrH2 (Me2CO) 2 (PPh3)2] BF4.
Heys JR, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 33(5), 431-438 (1993)
L R Hall et al.
The Journal of biological chemistry, 265(21), 12349-12355 (1990-07-25)
Liver microsomal cytochrome P-450 readily N-dealkylates N,N-dimethylamides. N-Methyl-N-hydroxymethyl amides were isolated as intermediates and characterized by gas chromatography-mass spectrometry as their trimethylsilyl ethers. Intramolecular kinetic deuterium isotope effects measured for the enzymic N-demethylation of a series of 12 aromatic and
Ji Young Kim et al.
Journal of controlled release : official journal of the Controlled Release Society, 152(1), 13-20 (2011-03-01)
Polymer micelles have been used widely for delivery of poorly water-soluble drugs. Such drug delivery, however, has been based primarily on hydrophobic interactions. For better drug loading and improved stability, hydrotropic polymer micelles were used. To develop a versatile polymer
Ji Young Kim et al.
Journal of pharmaceutical sciences, 99(9), 3953-3965 (2010-07-08)
The solubilizing ability of two aromatic hydrotropes, N,N-diethylnicotinamide (DENA) and N,N-dimethylbenzamide (DMBA), was investigated using a set of 13 poorly soluble, structurally diverse drugs. The number of aromatic rings in the solute molecule has a very strong effect on the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 276170-100G | 4061831829181 |
| 276170-25G | 4061826185445 |
| 276170-500G | 4061831829198 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service