All Photos(2)
About This Item
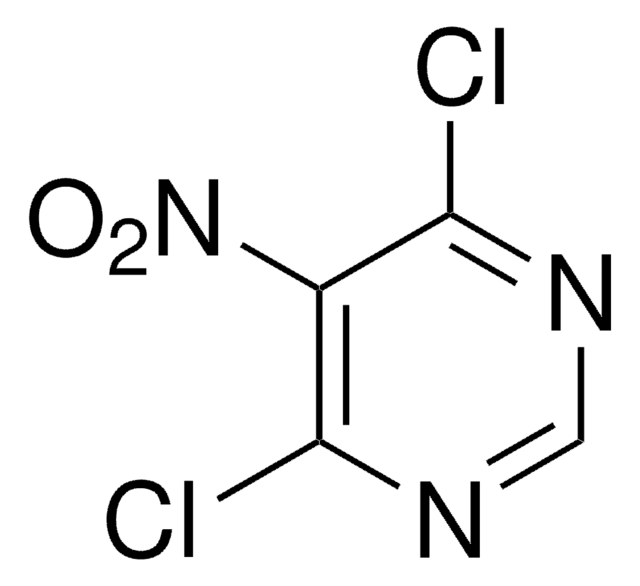
Empirical Formula (Hill Notation):
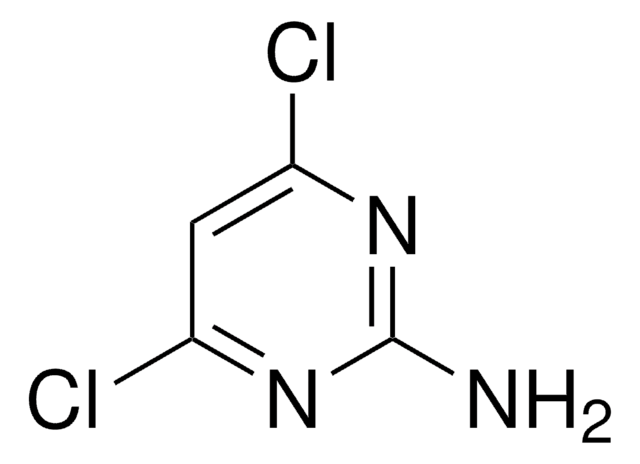
C4H3Cl2N3
CAS Number:
Molecular Weight:
163.99
Beilstein:
126885
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
mp
145-148 °C (lit.)
solubility
95% ethanol: soluble 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
functional group
chloro
storage temp.
2-8°C
SMILES string
Nc1c(Cl)ncnc1Cl
InChI
1S/C4H3Cl2N3/c5-3-2(7)4(6)9-1-8-3/h1H,7H2
InChI key
NIGDWBHWHVHOAD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Amino-4,6-dichloropyrimidine was used in the synthesis of:
- oxepane ring containing monocyclic, conformationally restricted bicyclic and spirocyclic nucleosides
- conformationally locked bicyclo[2.2.1]heptane/oxa-bicyclo[3.2.1]octane nucleosides
- N(7)-substituted purines
- chiral derivatives of (+)-erythro-9-(2-hydroxy-3-nonyl)adenine
- 9-alkyl-6-substituted-purine analogs, potent anticonvulsant agents
- pyrimido-oxazepines in a three-step process with microwave heating at 150°C
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G C Harriman et al.
Journal of medicinal chemistry, 35(22), 4180-4184 (1992-10-30)
The synthesis of various chiral derivatives of (+)-erythro-9-(2-hydroxy-3-nonyl)adenine, (+)-EHNA, from (2S,3R)-3-amino-1,2-O-isopropylidene-1,2-nonanediol by condensation with 5-amino-4,6-dichloropyrimidine is described. The compounds synthesized were C1'- and nor-C1'-(+)-EHNA derivatives. When tested with calf spleen ADA, C1'-OH- and nor-C1'-(+)-EHNA had comparable inhibitory activity that was
Jinglin Liu et al.
Journal of combinatorial chemistry, 8(3), 410-416 (2006-05-09)
A regiospecific strategy for the preparation of N(7)-substituted purines in an efficient manner was devised. This approach to 6,7,8-trisubstituted purines relies on the cyclization reactions of suitably substituted pyrimidines (1) with either a carboxylic acid or an aldehyde. The method
J L Kelley et al.
Journal of medicinal chemistry, 31(3), 606-612 (1988-03-01)
Several 9-alkyl-6-substituted-purines were synthesized and tested for anticonvulsant activity against maximal electroshock-induced seizures (MES) in rats. Most compounds were prepared in three steps from 5-amino-4,6-dichloropyrimidine or in two steps via alkylation of 6-chloropurine. Potent anticonvulsant activity against MES resided in
Tetrahedron Letters, 48, 1489-1489 (2007)
Sk Sahabuddin et al.
The Journal of organic chemistry, 71(16), 5980-5992 (2006-07-29)
The carbohydrate-derived substrate 3-C-allyl-1,2:5,6-di-O-isopropylidene-alpha-D-allofuranose was judiciously manipulated for preparing suitable synthons, which could be converted to a variety of isoxazolidino-spirocycles and -tricycles through the application of ring-closing metathesis (RCM) and intramolecular nitrone cycloaddition (INC) reactions. Cleavage of the isoxazolidine rings
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service