211311
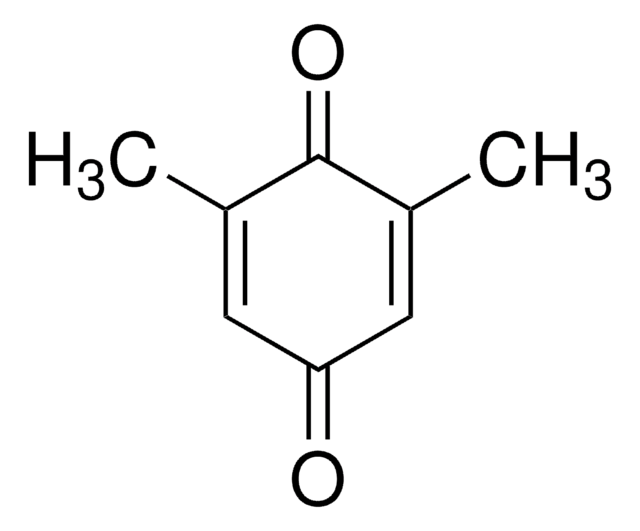
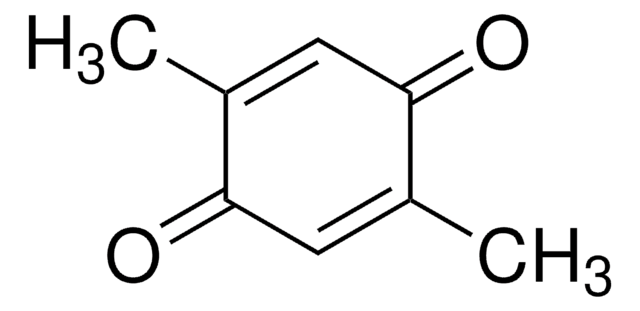
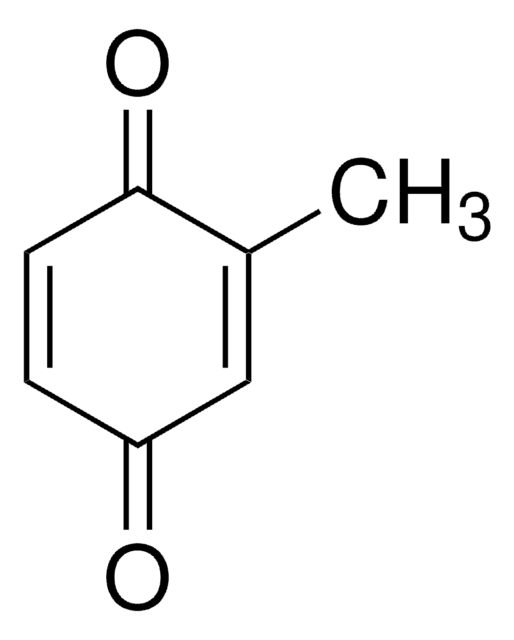
Methyl-p-benzoquinone
98%
Synonym(s):
p-Toluquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H3(=O)2
CAS Number:
Molecular Weight:
122.12
Beilstein:
471387
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
solid
mp
66-67 °C (lit.)
SMILES string
CC1=CC(=O)C=CC1=O
InChI
1S/C7H6O2/c1-5-4-6(8)2-3-7(5)9/h2-4H,1H3
InChI key
VTWDKFNVVLAELH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl-p-benzoquinone (MBQ) can be used as a coating that forms an interface between the electrode and lithium (Li) electrolyte for the fabrication of redox flow batteries. It can be reduced during positive electrospray ionization mass spectroscopy (ESI MS) and can be potentially used during corona discharge.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Michaela Bodner et al.
Chemoecology, 27(4), 171-175 (2017-08-15)
The defensive secretion of the julid diplopod
C L Blankespoor et al.
Parasitology, 115 ( Pt 1), 105-110 (1997-07-01)
The defensive glands of beetles, Tenebrio molitor, infected with metacestodes (cysticercoids) of Hymenolepis diminuta are everted less frequently upon stimulation, and contain less toluquinone (methylbenzoquinone) and m-cresol, than glands of uninfected controls. These differences, as shown in predation trials with
Jiying Pei et al.
Journal of the American Society for Mass Spectrometry, 28(11), 2454-2461 (2017-08-09)
Unexpected reduction of iminoquinone (IQ) and quinone derivatives was first reported during positive electrospray ionization mass spectrometry. Upon increasing spray voltage, the intensities of IQ and quinone derivatives decreased drastically, accompanying the increase of the intensities of the reduction products
N K Cénas et al.
Biochimica et biophysica acta, 767(1), 108-112 (1984-10-26)
The rate constants of NADH oxidation by quinones are increased with the oxidation potential increase: log kox (M-1 X s-1) = -0.25 + 12.2 E0(7) (V) for o-quinones and log kox (M-1 X s-1) = -3.06 + 13.5 E0(7) (V)
A I Vovk et al.
Ukrainskii biokhimicheskii zhurnal (1978), 65(4), 11-16 (1993-07-01)
Inactivation kinetics of pyruvate decarboxylase under joint action of substrate and substituted quinones in aqueous solutions which contain 1.0-13.5 vol.% of methyl alcohol has been investigated. The observed inactivation rate constant of pyruvate decarboxylase sharply decreases with the increase of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service