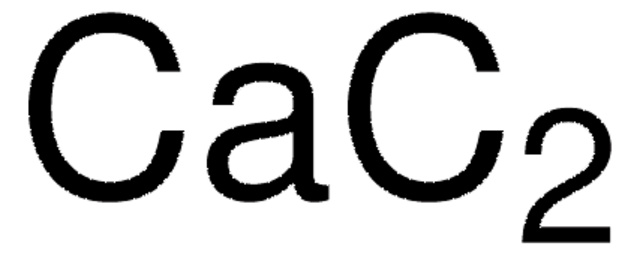21039
Calcium carbide
granulated, technical, ≥75% (gas-volumetric)
Synonym(s):
Calcium acetylide, Calcium dicarbide
About This Item
Recommended Products
grade
technical
Quality Level
Assay
≥75% (gas-volumetric)
form
pieces
quality
granulated
particle size
0.1-1 mm
density
2.22 g/mL at 25 °C (lit.)
SMILES string
[Ca++].[C-]#[C-]
InChI
1S/C2.Ca/c1-2;/q-2;+2
InChI key
UIXRSLJINYRGFQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- NUCLEOPHILIC ADDITION OF PHOSPHINOXIDES AND ALCOHOLS TO ACETYLENE GENERATED in situ FROM CALCIUM CARBIDE: This study discusses the reactivity of acetylene generated from calcium carbide for various organic synthesis applications (Aleksandrovna, 2024).
- Construction of 3-Methyl-2-Substituted Benzofurans and 3-Methyl-2-Substituted Benzothiophenes Using Solid Calcium Carbide as a Substitute for Gaseous …: Describes a synthetic method using solid calcium carbide in organic synthesis, highlighting its practicality and environmental benefits (Wang et al., 2024).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3 - Water-react 1
Target Organs
Respiratory system
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service